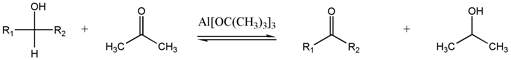Alcohol oxidation
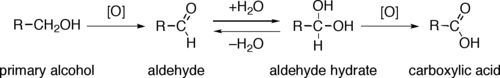
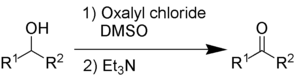
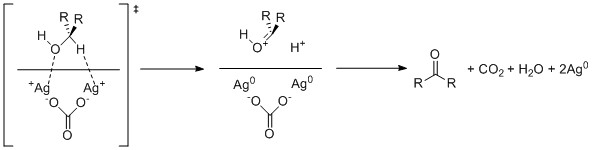
[2] Through a variety of mechanisms, the removal of a hydride equivalent converts a primary or secondary alcohol to an aldehyde or ketone, respectively.
The largest operations involve methanol and ethanol to formaldehyde and acetaldehyde, which are produced on million ton scale annually.
Other large scale aldehydes and ketones are produced by autoxidation or hydrocarbons: benzaldehyde from toluene, acrolein from propylene, acetone from cumene, cyclohexanone from cyclohexanol.
These salts are less reactive, more easily handled, and more selective than Collins reagent in oxidations of alcohols.
The related N-tert-Butylbenzenesulfinimidoyl chloride combines both the sulfur(IV), the base, and the activating Lewis acid in one molecule.
[2] In the laboratory, vicinal diols suffer oxidative breakage at a carbon-carbon bond with some oxidants such as sodium periodate (NaIO4), (diacetoxyiodo)benzene (PhI(OAc)2)[9] or lead tetraacetate (Pb(OAc)4), resulting in generation of two carbonyl groups.
Large scale oxidations of this type are used for the conversion of cyclohexanol alone or as a mixture with cyclohexanone to adipic acid.
[2] Many specialty reagents have been developed for laboratory scale oxidations of alcohols to carboxylic acids.
Potassium permanganate (KMnO4) oxidizes primary alcohols to carboxylic acids very efficiently.
For the reaction to proceed efficiently, the alcohol must be at least partially dissolved in the aqueous solution.
KMnO4 reacts with many functional groups, such as secondary alcohols, 1,2-diols, aldehydes, alkenes, oximes, sulfides and thiols, and carbon-carbon double bonds.
[17] The use of chlorites as terminal oxidants in conjunction with both hypochlorites and TEMPO gives carboxylic acids without chlorination side products.
In conjunction with Sharpless dihydroxylation, this method can be used to generate enantiopure α-hydroxy acids.