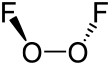Dioxygen difluoride
[2] Dioxygen difluoride can be obtained by subjecting a 1:1 mixture of gaseous fluorine and oxygen at low pressure (7–17 mmHg (0.9–2.3 kPa) is optimal) to an electric discharge of 25–30 mA at 2.1–2.4 kV.
[4] Another synthesis involves mixing O2 and F2 in a stainless steel vessel cooled to −196 °C (77.1 K), followed by exposing the elements to 3 MeV bremsstrahlung for several hours.
[5] All of these methods involve synthesis according to the equation It also arises from the thermal decomposition of ozone difluoride:[6] In O2F2, oxygen is assigned the unusual oxidation state of +1.
The structure of dioxygen difluoride resembles that of hydrogen peroxide, H2O2, in its large dihedral angle, which approaches 90° and C2 symmetry.
Even at a temperature of −160 °C (113 K), 4% decomposes each day[1] by this process: The other main property of this unstable compound is its oxidizing power, although most experimental reactions have been conducted near −100 °C (173 K).