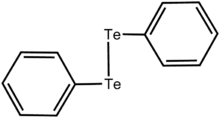
Diphenyl ditelluride
This orange-coloured solid is the oxidized derivative of the unstable benzenetellurol, PhTeH.
Ph2Te2 is used as a source of the PhTe unit in organic synthesis[2] and as a catalyst for redox reactions.
[3] The compound is a strong nucleophile, easily displacing halides.
It also adds electrophilically across multiple bonds, and traps radicals.
[4] Ph2Te2 is prepared by the oxidation of tellurophenolate, which is generated via the Grignard reagent:[5] The molecule has C2 symmetry.