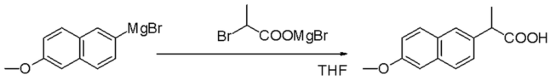
Grignard reagent
Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds.
For example, when reacted with another halogenated compound R'−X' in the presence of a suitable catalyst, they typically yield R−R' and the magnesium halide MgXX' as a byproduct; and the latter is insoluble in the solvents normally used.
Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques.
Traditionally Grignard reagents are prepared by treating an organic halide (normally organobromine) with magnesium metal.
Water and air, which rapidly destroy the reagent by protonolysis or oxidation, are excluded.
[2] As is common for reactions involving solids and solution, the formation of Grignard reagents is often subject to an induction period.
[3] Most organohalides will work, but carbon-fluorine bonds are generally unreactive, except with specially activated magnesium (through Rieke metals).
Typically the reaction to form Grignard reagents involves the use of magnesium ribbon.
Many methods have been developed to weaken this passivating layer, thereby exposing highly reactive magnesium to the organic halide.
Mechanical methods include crushing of the Mg pieces in situ, rapid stirring, and sonication.
Furthermore, the side-products are innocuous: The amount of Mg consumed by these activating agents is usually insignificant.
A small amount of mercuric chloride will amalgamate the surface of the metal, enhancing its reactivity.
[11] This method offers the advantage that the Mg transfer tolerates many functional groups.
This method has been used to make adamantane-based Grignard reagents, which are, due to C-C coupling side reactions, difficult to make by the conventional method from the alkyl halide and Mg.
The reductive transmetalation achieves:[13] Because Grignard reagents are so sensitive to moisture and oxygen, many methods have been developed to test the quality of a batch.
Typical tests involve titrations with weighable, anhydrous protic reagents, e.g. menthol in the presence of a color-indicator.
In cases where the Grignard reagent is adding to an aldehyde or a prochiral ketone, the Felkin-Anh model or Cram's Rule can usually predict which stereoisomer will be formed.
With easily deprotonated 1,3-diketones and related acidic substrates, the Grignard reagent RMgX functions merely as a base, giving the enolate anion and liberating the alkane RH.
[17] Grignard reagents serve as a base for non-protic substrates (this scheme does not show workup conditions, which typically includes water).
Grignard reagents react with 1,4-dioxane to give the diorganomagnesium compounds and insoluble coordination polymer MgX2(dioxane)2 and (R = organic group, X = halide): This reaction exploits the Schlenk equilibrium, driving it toward the right.
Grignard reagents react with organolithium compounds to give ate complexes (Bu = butyl):[20] Grignard reagents do not typically react with organic halides, in contrast with their high reactivity with other main group halides.
In the presence of metal catalysts, however, Grignard reagents participate in C-C coupling reactions.
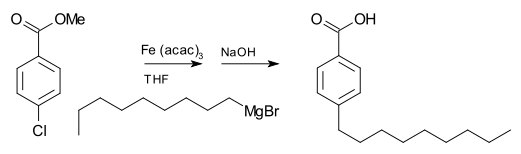
For example, nonylmagnesium bromide reacts with methyl p-chlorobenzoate to give p-nonylbenzoic acid, in the presence of Tris(acetylacetonato)iron(III) (Fe(acac)3), after workup with NaOH to hydrolyze the ester, shown as follows.
Without the Fe(acac)3, the Grignard reagent would attack the ester group over the aryl halide.
Additionally, an effective catalyst for the couplings of alkyl halides is the Gilman catalyst lithium tetrachlorocuprate (Li2CuCl4), prepared by mixing lithium chloride (LiCl) and copper(II) chloride (CuCl2) in THF.
The simple oxidation of Grignard reagents to give alcohols is of little practical importance as yields are generally poor.
In contrast, two-step sequence via a borane (vide supra) that is subsequently oxidized to the alcohol with hydrogen peroxide is of synthetic utility.
Adding just the Grignard and the alkene does not result in a reaction demonstrating that the presence of oxygen is essential.
In the Boord olefin synthesis, the addition of magnesium to certain β-haloethers results in an elimination reaction to the alkene.
An example of the Grignard reaction is a key step in the (non-stereoselective) industrial production of Tamoxifen[23] (currently used for the treatment of estrogen receptor positive breast cancer in women):[24]