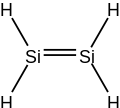
Disilene
However, unlike ethylene, disilene is kinetically unstable with respect to tautomerisation.
[1] Disilenes bearing sterically bulky substituents are isolable and have been well characterized although they remain mainly of academic interest.
The Si=Si distance in this molecule is 2.15 Å, about 10% shorter than a typical Si–Si single bond.
[2] Such species are typically prepared by reduction of organosilicon halides: An alternative synthesis involves photolysis of trisilacyclopropanes.
The disilene isomerizes to a tetracyclic compound by heating at 110 °C in xylene thereby releasing its strain energy[clarification needed].