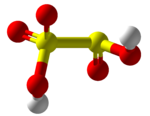
Disulfurous acid
The salts of disulfurous acid are called disulfites or metabisulfites.
[2] In contrast to disulfate (S2O2−7), disulfite has two directly connected sulfur atoms.
The oxidation state of the sulfur atom bonded to three oxygen atoms is +5 and its valence is 6, while that of the other sulfur is +3 and 4 respectively.
This article about theoretical chemistry is a stub.
You can help Wikipedia by expanding it.This article about an acid is a stub.