Disulfite
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S2O2−5.
[2] In contrast to disulfate (S2O2−7), disulfite ion (S2O2−5) has an unsymmetrical structure with an S-S bond.
[5] Although the equilibrium lies far to the left, evaporation of a bisulfite salt will produce a substantial amount of disulfite.
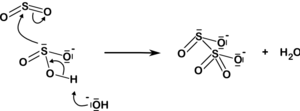
[6] Disulfite is the conjugate base of disulfurous acid (pyrosulfurous acid), which originates from sulfurous acid in accordance with the dehydration reaction above: The disulfite ion also arises from the addition of sulfur dioxide to the sulfite ion: Disulfite salts are used for preserving food and beverages and as antioxidants, with the main species used for this purpose being sodium metabisulfite (E223)[7] and potassium metabisulfite (E224).
[8] Sulfites are implicated in asthmatic reactions and may also cause symptoms in non-asthmatic individuals, namely dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea, and even life-threatening anaphylaxis.