Double layer (surface science)
The first layer, the surface charge (either positive or negative), consists of ions which are adsorbed onto the object due to chemical interactions.
It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored.
Interfacial DLs are most apparent in systems with a large surface-area-to-volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on the scale of micrometres to nanometres.
For instance, homogenized milk exists only because fat droplets are covered with a DL that prevents their coagulation into butter.
DLs exist in practically all heterogeneous fluid-based systems, such as blood, paint, ink and ceramic and cement slurry.
In 1853, he showed that an electrical double layer (DL) is essentially a molecular dielectric and stores charge electrostatically.
[3][4][5] This model, while a good foundation for the description of the interface, does not consider important factors including diffusion/mixing of ions in solution, the possibility of adsorption onto the surface, and the interaction between solvent dipole moments and the electrode.
Louis Georges Gouy in 1910 and David Leonard Chapman in 1913 both observed that capacitance was not a constant and that it depended on the applied potential and the ionic concentration.
In this model, the charge distribution of ions as a function of distance from the metal surface allows Maxwell–Boltzmann statistics to be applied.
The observation of long-distance inter-protein electron transfer through the aqueous solution[7] has been attributed to a diffuse region between redox partner proteins (cytochromes c and c1) that is depleted of cations in comparison to the solution bulk, thereby leading to reduced screening, electric fields extending several nanometers, and currents decreasing quasi exponentially with the distance at rate ~1 nm−1.
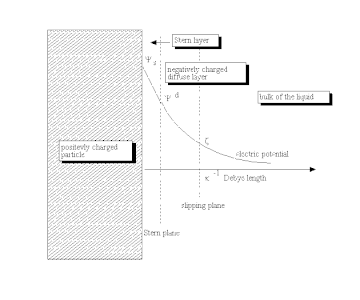
The Stern model has its own limitations, namely that it effectively treats ions as point charges, assumes all significant interactions in the diffuse layer are Coulombic, assumes dielectric permittivity to be constant throughout the double layer, and that fluid viscosity is constant plane.
[12] He proposed that some ionic or uncharged species can penetrate the Stern layer, although the closest approach to the electrode is normally occupied by solvent molecules.
The outer Helmholtz plane (OHP) passes through the centres of solvated ions at the distance of their closest approach to the electrode.
Bockris, M. A. V. Devanathan and Klaus Müller[14] proposed the BDM model of the double-layer that included the action of the solvent in the interface.
This first layer of solvent molecules displays a strong orientation to the electric field depending on the charge.
Further research with double layers on ruthenium dioxide films in 1971 by Sergio Trasatti and Giovanni Buzzanca demonstrated that the electrochemical behavior of these electrodes at low voltages with specific adsorbed ions was like that of capacitors.
[4] Between 1975 and 1980, Brian Evans Conway conducted extensive fundamental and development work on ruthenium oxide electrochemical capacitors.
In 1999, he coined the term supercapacitor to explain the increased capacitance by surface redox reactions with faradaic charge transfer between electrodes and ions.
The physical and mathematical basics of electron charge transfer absent chemical bonds leading to pseudocapacitance was developed by Rudolph A. Marcus.
[17] There are detailed descriptions of the interfacial DL in many books on colloid and interface science[18][19][20] and microscale fluid transport.
[21][22] There is also a recent IUPAC technical report[23] on the subject of interfacial double layer and related electrokinetic phenomena.
It yields a simple relationship between electric charge in the diffuse layer σd and the Stern potential Ψd:[28]
It yields the following expression for electric potential Ψ in the spherical DL as a function of the distance r from the particle center:
The primary difference between a double layer on an electrode and one on an interface is the mechanism of surface charge formation.
The formation of electrical double layer (EDL) has been traditionally assumed to be entirely dominated by ion adsorption and redistribution.
With considering the fact that the contact electrification between solid-solid is dominated by electron transfer, it is suggested by Wang that the EDL is formed by a two-step process.
Electron transfer occurs first to make the “neutral” atoms on solid surface become charged, i.e., the formation of ions.