Point of zero charge
This concept has been introduced in the studies dealing with colloidal flocculation to explain why pH is affecting the phenomenon.
Generally, the pzc in electrochemistry is the value of the negative decimal logarithm of the activity of the potential-determining ion in the bulk fluid.
For example, in the field of environmental science, it determines how easily a substrate is able to adsorb potentially harmful ions.
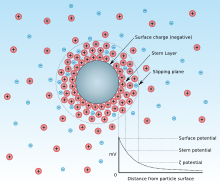
The pzc is the same as the isoelectric point (iep) if there is no adsorption of other ions than the potential determining H+/OH− at the surface[clarification needed].
The pzc is typically obtained by acid-base titrations of colloidal dispersions while monitoring the electrophoretic mobility of the particles and the pH of the suspension.
Once satisfactory curves are obtained (acid/base amount—pH, and pH—zeta potential), the pzc is established as the common intersection point (cip) of the lines.
[citation needed] In the field of environmental science, adsorption is involved in many techniques that can eliminate pollutants and governs the concentration of chemicals in soils and/or atmosphere.
When studying pollutant degradation or a sorption process, it is important to examine the pzc value related to adsorption.