DuPont Central Research
Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India (Hyderabad).
[1] The company established a tradition of basic scientific research starting with hiring of Wallace Carothers in 1928 and his systemization of polymer science that led to the development of polyamides such as nylon-6,6 and polychloroprene (neoprene) in the early 1930s.
The execution and publication of high quality research assisted recruiting and promoted the image of DuPont while raising morale among the CRD staff.
It was also accepted that every year a number of scientists would leave DuPont for academic positions and that several professors would join the staff permanently.
Jack Roberts of Caltech and Speed Marvel each consulted for well over 50 years and provided a steady supply of well-trained chemists.
The scientific accomplishments of Theodore L. Cairns, William D. Phillips, Earl Muetterties, Howard E. Simmons, Jr., and George Parshall were recognized by their election to the National Academy of Sciences.
Nonetheless, it remains difficult for a technician to break into the bench chemist ranks and they usually transfer to business units in search of more opportunity.
The issue was that they were too senior and naive to move into entry-level positions in businesses and their competition were similarly aged BS engineers who would have had about five years of experience keeping a plant running.
Responsibility for the technical direction of research has shifted to the chemist as they carry out short-term projects in support of the business units.
[6] Smart's comments in Chemical Reviews in 1996, “Scientific and commercial interests in fluorine chemistry burgeoned after 1980, largely fueled by the need to replace industrial chlorofluorocarbons and the rapidly growing practical opportunities for organofluorine compounds in crop protection, medicine and diverse materials applications.
CRD undertook a program on alternatives for chlorofluorocarbons in refrigerants in the late 1970s after the first warnings of damage to stratospheric ozone were published.
The Catalysis Center of CRD, under the leadership of Leo Manzer, was quick to respond with new technology to produce alternative hydrochlorofluorocarbons (HCFCs) that were commercialized as DuPont's Suva refrigerants.
Prospective applications included dyes, pharmaceuticals, pesticides, organic magnets, and incorporation in new types of polymers.
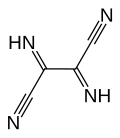
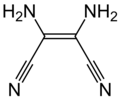
Another line of chemistry developed around Owen Webster's synthesis of diiminosuccinonitrile (DISN), which could be converted to diaminomaleonitrile (DAMN), leading to another series of patent and papers.
Arthur Sleight led a team focused on perovskites, such as the K-Bi-Pb-O system, that laid the groundwork for subsequent breakthroughs in high-temperature superconductors.
[8] Indicative of interplay between applications and fundamental science were many studies on stereodynamics conducted at CRD by Jesson, Meakin, and Muetterties.
[10] At about the same time, Andrew Janowicz developed a useful version of cobalt catalyzed chain transfer for controlling the molecular weight of free radicalpolymerizations.
Anthony Arduengo’s persistent carbenes opened up a new area of chemistry and they have proven to be important ligands in the metathesis process.
There was a vigorous effort on the activation of C-H bonds with contributions by Parshall, Thomas Herskovitz, Ittel, and David Thorn.
[17] Organometallic chemistry in CRD has further included R. Thomas Baker's heterobinuclear complexes, Patricia L. Watson's organolanthanides, William A. Nugent's metal-ligand multiple bonds,[18] Jeffery Thompson's and Mani Subramanyam's development of technetium complexes for radiopharmaceuticals, and Bob Burch's and Karin Karel's fluoro-organometallic chemistry.
DuPont developed a major technology based upon the nickel catalyzed addition of two molecules of hydrogen cyanide to butadiene, giving adiponitrile, a nylon intermediate, initially through the work of William C. Drinkard.
Other applications of homogeneous catalysis studied in CRD include ethylene polymerization, cyclohexane oxidation to adipic acid, and butadiene carbonylation to nylon intermediates.
These included printable electronics, thermal transfer methods for color filters, carbon nanotubes for field emission displays, and OLED materials and devices.
Charles Todd prepared substituted ureas as potential antibacterial agents, which when screened, proved to be effective herbicides.
In the mid- 1950s, CRD began work on the chemistry of nitrogen fixation in plants, a study that would develop into a major effort over the next decade.
In 1963, Ralph Hardy joined the CRD and brought Du Pont's nitrogen fixation research to international prominence with more than a hundred papers on the subject.
Fermentation microbiology and selective genetic modification became important to the CRD development of a biological route to 1,3-propylene glycol a new monomer for making polyester.
Substantial success was also achieved in the synthesis of unnatural peptides and proteins to accomplish specific functions and prediction of their tertiary structures.
This technology has led to significant improvements in the safety of the food supply chain in the United States and around the world.