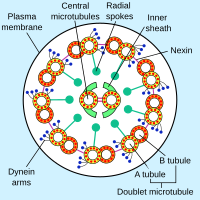
Dynein
Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella.
[1] It also helps transport cargo needed for cell function such as vesicles made by the endoplasmic reticulum, endosomes, and lysosomes (Karp, 2005).
[4] Cytoplasmic dynein positions the spindle at the site of cytokinesis by anchoring to the cell cortex and pulling on astral microtubules emanating from centrosome.
While a postdoctoral student at MIT, Tomomi Kiyomitsu discovered how dynein has a role as a motor protein in aligning the chromosomes in the middle of the cell during the metaphase of mitosis.
[5][6][7][8] Budding yeast have been a powerful model organism to study this process and has shown that dynein is targeted to plus ends of astral microtubules and delivered to the cell cortex via an offloading mechanism.
One projection, the coiled-coil stalk, binds to and "walks" along the surface of the microtubule via a repeated cycle of detachment and reattachment.
[14] As a result, the MTBD of dynein enters a low-affinity state, allowing the motor to move to new binding sites.
[18] Upon the release of the phosphate, the MTBD returns to a high affinity state and rebinds the MT, triggering the power stroke.
[23] The linker returns to a straight conformation and swings back to AAA5 from AAA2[24][25] and creates a lever-action,[26] producing the greatest displacement of dynein achieved by the power stroke[18] The cycle concludes with the release of ADP, which returns the AAA domain ring back to the “open” configuration.
[27] The light intermediate chain, which is a member of the Ras superfamily, mediates the attachment of several cargo adaptors to the dynein motor.
[36] Axonemal dyneins come in multiple forms that contain either one, two or three non-identical heavy chains (depending upon the organism and location in the cilium).
Groups of dynein molecules responsible for movement in opposite directions are probably activated and inactivated in a coordinated fashion so that the cilia or flagella can move back and forth.
The heavy chains of inner and outer arms of axonemal dynein are phosphorylated/dephosphorylated to control the rate of microtubule sliding.
Calcium fluctuations play an important role in altering cilia waveform and flagellar beat frequency (King, 2012).
Proper segregation is essential for producing haploid meiotic products with a normal complement of chromosomes.