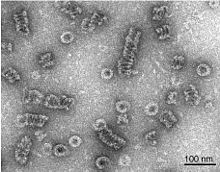
Dynamin
Members of the dynamin family are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface (particularly caveolae internalization) as well as at the Golgi apparatus.
[1][2][3] Dynamin family members also play a role in many processes including division of organelles,[4] cytokinesis and microbial pathogen resistance.
Dynamin has been extensively studied in the context of clathrin-coated vesicle budding from the cell membrane.
[7][8] The polymer constricts the underlying membrane upon GTP binding and hydrolysis via conformational changes emanating from the flexible neck region that alters the overall helical symmetry.
Mutations in Dynamin II have been found to cause dominant intermediate Charcot-Marie-Tooth disease.