Embryonic diapause
In more than 130 types of mammals where this takes place, the process occurs at the blastocyst stage of embryonic development,[1] and is characterized by a dramatic reduction or complete cessation of mitotic activity, arresting most often in the G0 or G1 phase of division.
[2] In placental embryonic diapause, the blastocyst does not immediately implant in the uterus after sexual reproduction has resulted in the zygote, but rather remains in this non-dividing state of dormancy until conditions allow for attachment to the uterine wall to proceed as normal.
Organisms which undergo embryonic diapause are able to synchronize the birth of offspring to the most favorable conditions for reproductive success, irrespective of when mating took place.
[3] Embryonic diapause is a relatively widespread phenomenon outside of mammals, with known occurrence in the reproductive cycles of many insects, nematodes, fish, and other non-mammalian vertebrates.
This may be evidence for the evolutionary significance of this phenomenon, with latent capacity for diapause potentially present in a much wider segment of species than known to occur naturally.
Regulation of the cell cycle as it relates to embryonic diapause has been linked to the dacapo gene in the fruit fly, responsible for inhibiting the formation of cyclin E-cdk2 complexes necessary for DNA synthesis.
Other studies have demonstrated, inversely, the lack of involvement of more common regulators of the cell cycle such as p53 within the placental model of embryonic diapause.
[11] Specifically within placental embryonic diapause, this cessation is led by the intentional failure of the blastocyst to implant in the uterine wall, which is an essential component in developmental progression in these species.
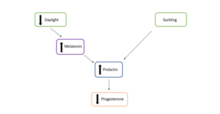
[11] Facultative diapause is regulated by several factors, including the maternal environment and ovarian competency, the pituitary gland, and metabolic stress and lactation.
The corpus luteum is a temporary endocrine organ that is formed from the leftover cells from the ovarian follicle in the ovary, once it has released a mature ovum.
[11] Similarly to facultative diapause, a series of hormonal changes arrest the blastocyst development, prior to implantation, preventing continued growth of the embryo.
[3] Both diapausing blastocysts and ESCs have transcriptome profile similarities, including downregulation of metabolism, biosynthesis and gene expression pathways.