Melatonin
[1] Its discovery in 1958 by Aaron B. Lerner and colleagues stemmed from the isolation of a substance from the pineal gland of cows that could induce skin lightening in common frogs.
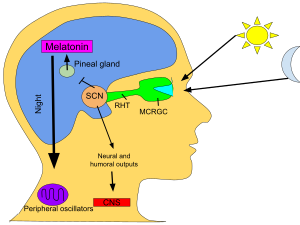
This compound was later identified as a hormone secreted in the brain during the night, playing a crucial role in regulating the sleep-wake cycle, also known as the circadian rhythm, in vertebrates.
[12] Furthermore, melatonin functions as a high-capacity antioxidant, or free radical scavenger, within mitochondria, playing a dual role in combating cellular oxidative stress.
This increase in antioxidant enzyme expression is mediated through signal transduction pathways activated by the binding of melatonin to its receptors.
Through these mechanisms, melatonin protects the cell against oxidative stress in two ways, and plays other roles in human health than only regulating the sleep-wake cycle.
[19] The establishment of regular melatonin levels in human infants occurs around the third month after birth, with peak concentrations observed between midnight and 8:00 am.
[14][15][16][17][18] Furthermore, the interaction of melatonin with reactive oxygen and nitrogen species results in the formation of metabolites capable of reducing free radicals.
[12][18] These metabolites, including cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK), contribute to the broader antioxidative effects of melatonin through further redox reactions with free radicals.
[citation needed] The efficacy of melatonin in disease treatment has been the subject of limited trials, with most available data deriving from small-scale, preliminary studies.
Preclinical investigations suggest that melatonin may augment cytokine production and promote the expansion of T cells,[29] thereby potentially mitigating acquired immunodeficiencies.
[30] Melatonin's potential to regulate weight gain is posited to involve its inhibitory effect on leptin, a hormone that serves as a long-term indicator of the body's energy status.
The biosynthesis of melatonin in animals involves a sequence of enzymatic reactions starting with L-tryptophan, which can be synthesized through the shikimate pathway from chorismate, found in plants, or obtained from protein catabolism.
The initial step in the melatonin biosynthesis pathway is the hydroxylation of L-tryptophan's indole ring by the enzyme tryptophan hydroxylase, resulting in the formation of 5-hydroxytryptophan (5-HTP).
[34] In bacteria, protists, fungi, and plants, the synthesis of melatonin also involves tryptophan as an intermediate but originates indirectly from the shikimate pathway.
[37] The mechanism of melatonin biosynthesis initiates with the hydroxylation of L-tryptophan, a process that requires the cofactor tetrahydrobiopterin (THB) to react with oxygen and the active site iron of tryptophan hydroxylase.
[43] Norepinephrine increases the concentration of intracellular cAMP via beta-adrenergic receptors, which in turn activates the cAMP-dependent protein kinase A (PKA).
[58] Anecdotal reports and formal research studies over the past few decades have established a link between melatonin supplementation and more vivid dreams.
[59] Melatonin's discovery is linked to the study of skin color changes in some amphibians and reptiles, a phenomenon initially observed through the administration of pineal gland extracts.
[60][61] In 1917, Carey Pratt McCord and Floyd P. Allen found that feeding extracts from the pineal glands of cows caused the skin of tadpoles to lighten by contracting the dark epidermal melanophores.
[65] The first patent for the therapeutic use of melatonin as a low-dose sleep aid was awarded to Richard Wurtman at the Massachusetts Institute of Technology in 1995.
[71] Melatonin was thus aptly named to reflect its role in preventing the darkening of the skin, highlighting the intersection of biochemistry and linguistics in scientific discovery.
Known as "the hormone of darkness", the onset of melatonin at dusk promotes activity in nocturnal (night-active) animals and sleep in diurnal ones including humans.
[78] In animals including humans,[79] the profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter.
In sheep, melatonin administration has also shown antioxidant and immune-modulatory regime in prenatally stressed offspring helping them survive the crucial first days of their lives.
[36][85] Notably high melatonin concentrations have been measured in popular beverages such as coffee, tea, wine, and beer, and crops including corn, rice, wheat, barley, and oats.
Only limited evidence of endogenous circadian rhythms in melatonin levels has been demonstrated in some plant species and no membrane-bound receptors analogous to those known in animals have been described.
There is no report of its occurrence in archaea which indicates that melatonin originated in bacteria[11] most likely to prevent the first cells from the damaging effects of oxygen in the primitive Earth's atmosphere.
[98] Naturally-occurring melatonin has been reported in foods including tart cherries to about 0.17–13.46 ng/g,[99] bananas, plums, grapes, rice, cereals, herbs,[100] olive oil, wine,[101] and beer.