Equilibrium chemistry
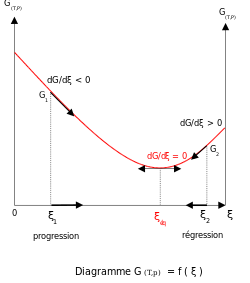
Chemical equilibrium is a dynamic state in which forward and backward reactions proceed at such rates that the macroscopic composition of the mixture is constant.
The potential, μi, of the ith species in a chemical reaction is the partial derivative of the free energy with respect to the number of moles of that species, Ni: A general chemical equilibrium can be written as[note 1] nj are the stoichiometric coefficients of the reactants in the equilibrium equation, and mj are the coefficients of the products.
This shows that when the reaction is exothermic (ΔHo, the standard enthalpy change, is negative), then K decreases with increasing temperature, in accordance with Le Châtelier's principle.
Formation of ammonia is also favoured by high pressure, as the volume decreases when the reaction takes place.
The same reaction, nitrogen fixation, occurs at ambient temperatures in nature, when the catalyst is an enzyme such as nitrogenase.
Gas-phase equilibria occur during combustion and were studied as early as 1943 in connection with the development of the V2 rocket engine.
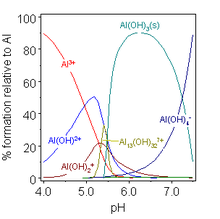
[16] Thus, the importance of equilibrium constants lies in the fact that, once their values have been determined by experiment, they can be used to calculate the concentrations, known as the speciation, of mixtures that contain the relevant species.
A broader definition of acid dissociation includes hydrolysis, in which protons are produced by the splitting of water molecules.
The general equilibrium can be written as The study of these complexes is important for supramolecular chemistry[24][25] and molecular recognition.
The objective of these studies is often to find systems with a high binding selectivity of a host (receptor) for a particular target molecule or ion, the guest or ligand.
For example, In aqueous solutions, metal ions will be present as aquo ions, so the reaction for the formation of the first complex could be written as[note 5] However, since water is in vast excess, the concentration of water is usually assumed to be constant and is omitted from equilibrium constant expressions.
[note 4] For the equilibrium a stability constant can be defined as follows:[28][29] The definition can easily be extended to include any number of reagents.
In biochemistry, an oxygen molecule can bind to an iron(II) atom in a heme prosthetic group in hemoglobin.
A better representation would be as this shows that when hydrogen ion concentration increases the equilibrium is shifted to the left in accordance with Le Châtelier's principle.
Hydrogen ion concentration can be increased by the presence of carbon dioxide, which behaves as a weak acid.
Therefore: For any half-reaction, the redox potential of an actual mixture is given by the generalized expression[note 6] This is an example of the Nernst equation.
Using these values, the actual electrode potential for a redox couple can be calculated as a function of the ratio of concentrations.
When another salt is present that has an ion in common, the common-ion effect comes into play, reducing the solubility of the primary solute.
Partition coefficients are very important in pharmacology because they determine the extent to which a substance can pass from the blood (an aqueous solution) through a cell wall which is like an organic solvent.
They are usually measured using water and octanol as the two solvents, yielding the so-called octanol-water partition coefficient.
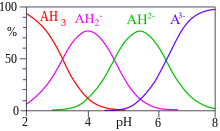
Such a compound may exist with a different extent of protonation depending on pH and the acid dissociation constant.
In its simplest form a reaction is performed in an organic solvent and unwanted by-products are removed by extraction into water at a particular pH.
The ligand, La−, forms a complex with the metal ion, Mb+, [MLx](b−ax)+ which has a strongly hydrophobic outer surface.
In the PUREX process, which is commonly used in nuclear reprocessing, uranium(VI) is extracted from strong nitric acid as the electrically neutral complex [UO2(TBP)2(NO3)2].
The strong nitric acid provides a high concentration of nitrate ions which pushes the equilibrium in favour of the weak nitrato complex.
Separation is achieved because the stability constant for the formation of the TBP complex increases as the size of the lanthanoid ion decreases.
An instance of ion-pair extraction is in the use of a ligand to enable oxidation by potassium permanganate, KMnO4, in an organic solvent.
When a ligand, such as a crown ether is added to an aqueous solution of KMnO4, it forms a hydrophobic complex with the potassium cation which allows the uncharged ion pair [KL]+[MnO4]− to be extracted into the organic solvent.
A distribution constant, Kd can be defined as where as and am are the equilibrium activities in the stationary and mobile phases respectively.
It can be shown that the rate of migration, ν, is related to the distribution constant by f is a factor which depends on the volumes of the two phases.