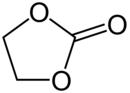
Ethylene carbonate
At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water.
[10] Ethylene carbonate was a universal component of an electrolyte in earlier (prior to ca.
2010) lithium-ion batteries, since it is responsible for the formation of the solid electrolyte interphase on the anode.
As dimethylcarbonate and other dialkylcarbonates became commercially available, they replaced ethylene carbonate in some modern lithium-ion batteries.
Photochlorination gives the tetrachloroethylene carbonate:[12] The tetrachloride is degraded to oxalyl chloride by amine catalysts.