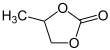
Propylene carbonate
[7] It has a high molecular dipole moment (4.9 D), considerably higher than those of acetone (2.91 D) and ethyl acetate (1.78 D).
[1] It is possible, for example, to obtain potassium, sodium, and other alkali metals by electrolysis of their chlorides and other salts dissolved in propylene carbonate.
[8] Due to its high relative permittivity (dielectric constant) of 64, it is frequently used as a high-permittivity component of electrolytes in lithium batteries, usually together with a low-viscosity solvent (e.g. dimethoxyethane).
[5] In electrospray ionization mass spectrometry, propylene carbonate is doped into low surface tension solutions to increase analyte charging.
[14] In the US, propylene carbonate is not regulated as a volatile organic compound (VOC) because it does not contribute significantly to the formation of smog and because its vapor is not known or suspected to cause cancer or other toxic effects.