Ferrocene
The discovery of ferrocene and its many analogues, known as metallocenes, sparked excitement and led to a rapid growth in the discipline of organometallic chemistry.
Ferrocene itself has no large-scale applications, but has found more niche uses in catalysis, as a fuel additive, and as a tool in undergraduate education.
The first known synthesis may have been made in the late 1940s by unknown researchers at Union Carbide, who tried to pass hot cyclopentadiene vapor through an iron pipe.
Years later, a sample of the sludge that had been saved was obtained and analyzed by Eugene O. Brimm, shortly after reading Kealy and Pauson's article, and was found to consist of ferrocene.
[8] In 1951, Peter L. Pauson and Thomas J. Kealy at Duquesne University attempted to prepare fulvalene ((C5H4)2) by oxidative dimerization of cyclopentadiene (C5H6).
To that end, they reacted the Grignard compound cyclopentadienyl magnesium bromide in diethyl ether with ferric chloride as an oxidizer.
[13] Robert Burns Woodward, Geoffrey Wilkinson, et al. deduced observe that the compound was diamagnetic and nonpolar.
[18] Philip Frank Eiland and Raymond Pepinsky confirmed the structure through X-ray crystallography and later by NMR spectroscopy.
Application of molecular orbital theory with the assumption of a Fe2+ centre between two cyclopentadienide anions C5H−5 resulted in the successful Dewar–Chatt–Duncanson model, allowing correct prediction of the geometry of the molecule as well as explaining its remarkable stability.
[29] In solution, eclipsed D5h ferrocene was determined to dominate over the staggered D5d conformer, as suggested by both Fourier-transform infrared spectroscopy and DFT calculations.
[8] Another early synthesis of ferrocene was by Miller et al.,[9] who treated metallic iron with gaseous cyclopentadiene at elevated temperature.
[32] More efficient preparative methods are generally a modification of the original transmetalation sequence using either commercially available sodium cyclopentadienide[33] or freshly cracked cyclopentadiene deprotonated with potassium hydroxide[34] and reacted with anhydrous iron(II) chloride in ethereal solvents.
Modern modifications of Pauson and Kealy's original Grignard approach are known: Even some amine bases (such as diethylamine) can be used for the deprotonation, though the reaction proceeds more slowly than when using stronger bases:[33] Direct transmetalation can also be used to prepare ferrocene from some other metallocenes, such as manganocene:[35] Ferrocene is an air-stable orange solid with a camphor-like odor.
[36] As expected for a symmetric, uncharged species, ferrocene is soluble in normal organic solvents, such as benzene, but is insoluble in water.
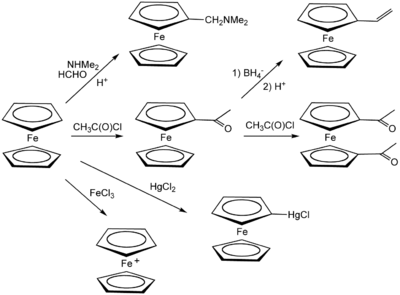
[37][38] Ferrocene undergoes many reactions characteristic of aromatic compounds, enabling the preparation of substituted derivatives.
A common undergraduate experiment is the Friedel–Crafts reaction of ferrocene with acetic anhydride (or acetyl chloride) in the presence of phosphoric acid as a catalyst.
[41] In the presence of aluminium chloride, Me2NPCl2 and ferrocene react to give ferrocenyl dichlorophosphine,[42] whereas treatment with phenyldichlorophosphine under similar conditions forms P,P-diferrocenyl-P-phenyl phosphine.
[48] The remarkably reversible oxidation-reduction behaviour has been extensively used to control electron-transfer processes in electrochemical[49][50] and photochemical[51][52] systems.
[62] Ferrocene derivatives have been investigated as drugs,[63] with one compound ferrocerone [ru] approved for use in the USSR in the 1970s as an iron supplement, though it is no longer marketed today.
Ferrocene and related derivatives are used as powerful burn rate catalysts in ammonium perchlorate composite propellant.
[80] X-ray diffraction analysis of this compound confirms that the cyclopentadienyl ligands are not co-planar with the benzene core but have alternating dihedral angles of +30° and −80°.
The synthesis of hexaferrocenylbenzene has been reported using Negishi coupling of hexaiodidobenzene and diferrocenylzinc, using tris(dibenzylideneacetone)dipalladium(0) as catalyst, in tetrahydrofuran:[80] The yield is only 4%, which is further evidence consistent with substantial steric crowding around the arene core.
In addition to using organic polymer backbones, these pendant ferrocene units have been attached to inorganic backbones such as polysiloxanes, polyphosphazenes, and polyphosphinoboranes, (–PH(R)–BH2–)n, and the resulting materials exhibit unusual physical and electronic properties relating to the ferrocene / ferrocinium redox couple.
[81] Both PVFc and PFcMA have been tethered onto silica wafers and the wettability measured when the polymer chains are uncharged and when the ferrocene moieties are oxidised to produce positively charged groups.
The contact angle with water on the PFcMA-coated wafers was 70° smaller following oxidation, while in the case of PVFc the decrease was 30°, and the switching of wettability is reversible.
In the PFcMA case, the effect of lengthening the chains and hence introducing more ferrocene groups is significantly larger reductions in the contact angle upon oxidation.