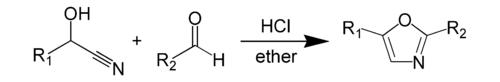
Fischer oxazole synthesis
A more specific example of Fischer oxazole synthesis involves reacting mandelic acid nitrile with benzaldehyde to give 2,5-diphenyl-oxazole.
[4] The Fischer oxazole synthesis is a type of dehydration reaction which can occur under mild conditions in a rearrangement of the groups that would not seem possible.
The product, which is the 2,5-disubstituted oxazole, precipitates as the hydrochloride and can be converted to the free base by the addition of water or by boiling with alcohol.
The cyanohydrin abstracts the hydrogen from HCl while the chloride ion attacks the carbon in the cyano group.
[4]Diarylazoles are common structural motifs in both natural products and drug candidates, however they are difficult to synthesize.
[6] Another useful example is the one pot two-step synthesis of halfordinol, a parent compound for Rutaceae alkaloids.