Flame
[note 1] In this state they can then readily react with oxygen in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame.
Sufficient energy in the flame will excite the electrons in some of the transient reaction intermediates such as the methylidyne radical (CH) and diatomic carbon (C2), which results in the emission of visible light as these substances release their excess energy (see spectrum below for an explanation of which specific radical species produce which specific colors).
For instance, a well-known chemical kinetics scheme, GRI-Mech,[6] uses 53 species and 325 elementary reactions to describe combustion of biogas.
The colder part of a diffusion (incomplete combustion) flame will be red, transitioning to orange, yellow, and white as the temperature increases as evidenced by changes in the black-body radiation spectrum.
The transitions are often apparent in fires, in which the color emitted closest to the fuel is white, with an orange section above it, and reddish flames the highest of all.
[8] Specific colors can be imparted to the flame by introduction of excitable species with bright emission spectrum lines.
pressure): Dicyanoacetylene, a compound of carbon and nitrogen with chemical formula C4N2 burns in oxygen with a bright blue-white flame at a temperature of 5,260 K (4,990 °C; 9,010 °F), and at up to 6,000 K (5,730 °C; 10,340 °F) in ozone.
[10] This high flame temperature is partially due to the absence of hydrogen in the fuel (dicyanoacetylene is not a hydrocarbon) thus there is no water among the combustion products.
The process depends on a fine balance of temperature and concentration of the reacting mixture, and if conditions are right it can initiate without any external ignition source.
In microgravity or zero gravity environment, such as in orbit, natural convection no longer occurs and the flame becomes spherical, with a tendency to become bluer and more efficient.
There are several possible explanations for this difference, of which the most likely is the hypothesis that the temperature is sufficiently evenly distributed that soot is not formed and complete combustion occurs.

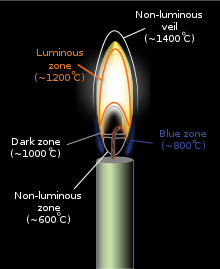
The interior of the luminous zone can be much hotter, beyond 1,500 °C (2,730 °F). [ 3 ]



