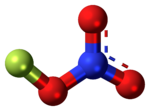
Fluorine nitrate
Fluorine nitrate is an inert molecule thought to play a significant role in atmospheric chemistry.
[2] In 1935, professor George H. Cady was first to synthesize fluorine nitrate and has since maintained a long and controversial history.
[3] In a 1995 study performed by Universität Tubingen in Germany, found through electron diffraction that the nitrogen–oxygen bond is surprisingly long at about a length of 150.7 ppm.
[5] The relationship between the ionization potential and the highest occupied molecular orbital (HOMO) in fluorine nitrate was determined to be large.
[5] Despite the molecule’s inert nature, it is asserted by the 1996 study that fluorine nitrate may be the best possible reservoir species in the process of ozone depletion.