Fluorescence spectroscopy
[1] The molecule then drops down to one of the various vibrational levels of the ground electronic state again, emitting a photon in the process.
[1] As molecules may drop down into any of several vibrational levels in the ground state, the emitted photons will have different energies, and thus frequencies.
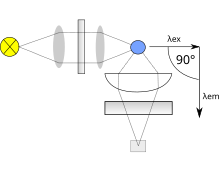
Both types use the following scheme: the light from an excitation source passes through a filter or monochromator, and strikes the sample.
A laser only emits light of high irradiance at a very narrow wavelength interval, typically under 0.01 nm, which makes an excitation monochromator or filter unnecessary.
This results in a better signal-to-noise ratio, and lowers the detection limit by approximately a factor 10000,[3] when compared to the 180° geometry.
To correct this, a beam splitter can be applied after the excitation monochromator or filter to direct a portion of the light to a reference detector.
Two other topics that must be considered include the optics used to direct the radiation and the means of holding or containing the sample material (called a cuvette or cell).
In both cases, it is important to select materials that have relatively little absorption in the wavelength range of interest.
Correction of all these instrumental factors for getting a ‘standard’ spectrum is a tedious process, which is only applied in practice when it is strictly necessary.
This is the case when measuring the quantum yield or when finding the wavelength with the highest emission intensity for instance.
Raman scattering is the result of a virtual electronic state induced by the excitation light.
Reabsorption happens because another molecule or part of a macromolecule absorbs at the wavelengths at which the fluorophore emits radiation.
Another inner filter effect occurs because of high concentrations of absorbing molecules, including the fluorophore.
Resultingly, only a small percentage of the excitation light reaches the fluorophores that are visible for the detection system.
Typically, tryptophan has a wavelength of maximum absorption of 280 nm and an emission peak that is solvatochromic, ranging from ca.
When performing experiments with denaturants, surfactants or other amphiphilic molecules, the microenvironment of the tryptophan might change.
For example, if a protein containing a single tryptophan in its 'hydrophobic' core is denatured with increasing temperature, a red-shifted emission spectrum will appear.
This is due to the exposure of the tryptophan to an aqueous environment as opposed to a hydrophobic protein interior.
Fluorescence spectroscopy is used in, among others, biochemical, medical, and chemical research fields for analyzing organic compounds.
Atomic Fluorescence Spectroscopy (AFS) techniques are useful in other kinds of analysis/measurement of a compound present in air or water, or other media, such as CVAFS which is used for heavy metals detection, such as mercury.
[14] Recent advances in computer science and machine learning have even enabled detection of bacterial contamination of water.
[16] Fluorescence spectroscopy in biophysical research enables individuals to visualize and characterize lipid domains within cellular membranes.