Standard enthalpy of formation
The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K).
[2] For example, the standard enthalpy of formation of carbon dioxide is the enthalpy of the following reaction under the above conditions: All elements are written in their standard states, and one mole of product is formed.
The standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole (kJ mol−1), but also in kilocalorie per mole, joule per mole or kilocalorie per gram (any combination of these units conforming to the energy per mass or amount guideline).
All elements in their reference states (oxygen gas, solid carbon in the form of graphite, etc.)
For tabulation purposes, standard formation enthalpies are all given at a single temperature: 298 K, represented by the symbol ΔfH⦵298 K. For many substances, the formation reaction may be considered as the sum of a number of simpler reactions, either real or fictitious.
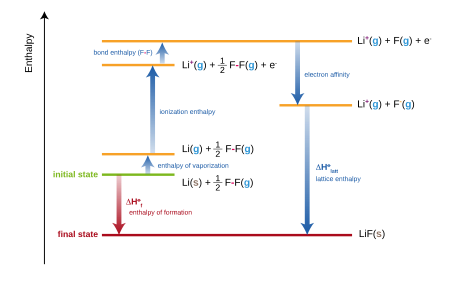
For ionic compounds, the standard enthalpy of formation is equivalent to the sum of several terms included in the Born–Haber cycle.
The equation is therefore rearranged to evaluate the lattice energy:[3] The formation reactions for most organic compounds are hypothetical.
However the standard enthalpy of combustion is readily measurable using bomb calorimetry.
The combustion of methane: is equivalent to the sum of the hypothetical decomposition into elements followed by the combustion of the elements to form carbon dioxide (CO2) and water (H2O): Applying Hess's law, Solving for the standard of enthalpy of formation, The value of
The negative sign shows that the reaction, if it were to proceed, would be exothermic; that is, methane is enthalpically more stable than hydrogen gas and carbon.
A given reaction is considered as the decomposition of all reactants into elements in their standard states, followed by the formation of all products.
This calculation has a tacit assumption of ideal solution between reactants and products where the enthalpy of mixing is zero.
, and the heat of reaction is simplified to which is the equation in the previous section for the enthalpy of combustion