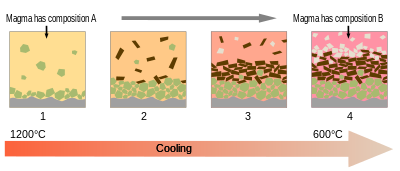
Fractional crystallization (geology)
[5] For example, the partial pressure (fugacity) of water in silicate melts can be of prime importance, as in near-solidus crystallization of magmas of granite composition.
[9][10] Experiments have provided many examples of the complexities that control which mineral is crystallized first as the melt cools down past the liquidus.
MgO and SiO2 concentrations in melts are among the variables that determine whether forsterite olivine or enstatite pyroxene is precipitated,[11] but the water content and pressure are also important.
[12] Granitic magmas provide additional examples of how melts of generally similar composition and temperature, but at different pressure, may crystallize different minerals.
[citation needed] Experimentally-determined phase diagrams for simple mixtures provide insights into general principles.