Frank-Kamenetskii theory
In combustion, Frank-Kamenetskii theory explains the thermal explosion of a homogeneous mixture of reactants, kept inside a closed vessel with constant temperature walls.
It is named after a Russian scientist David A. Frank-Kamenetskii, who along with Nikolay Semenov developed the theory in the 1930s.
During the initial period of ignition, the consumption of reactant concentration is negligible (see
is the amount of heat released per unit mass of fuel consumed, and a reaction rate governed by Arrhenius law, the energy equation becomes where An increment in temperature of order
is the Frank-Kamenetskii temperature is enough to increase the chemical reaction by amount
,[10] as is evident from the ratio[11] Non-dimensional scales of time, temperature, length, and heat transfer may be defined as where Substituting the non-dimensional variables in the energy equation from the introduction Since
Before Frank-Kamenetskii, his doctoral advisor Nikolay Semyonov (or Semenov) proposed a thermal explosion theory with a simpler model with which he assumed a linear function for the heat conduction process instead of the Laplacian operator.
The relevant importance between the two terms are determined by the Damköhler number
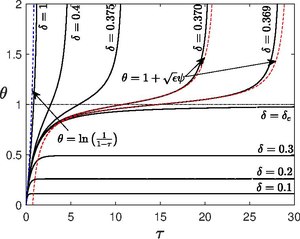
, the linear term eventually dominates and the system is able to reach a steady state as
From the properties of Lambert W function, it is easy to see that the steady state temperature provided by the above equation exists only when
is called as Frank-Kamenetskii parameter as a critical point where the system bifurcates from the existence of steady state to explosive state at large times.
, the system explodes since the exponential term dominates as time proceeds.
can be neglected in which case the problem admits an explicit solution, At time
This time is also referred to as the adiabatic induction period since the heat conduction term
, the system takes very long time to explode.
The analysis for this limit was first carried out by Frank-Kamenetskii.,[12] although proper asymptotics were carried out only later by D. R. Kassoy and Amable Liñán[13] including reactant consumption because reactant consumption is not negligible when
into the governing equation and collect only the leading-order terms to find out where the boundary condition is derived by matching with the initial region wherein
The solution to the above-mentioned problem is given by which immediately reveals that
Sources:[14][15] The only parameter which characterizes the explosion is the Damköhler number
is very low, heat conduction time is much faster than the chemical reaction time, such that all the heat produced by the chemical reaction is immediately conducted to the wall, thus there is no explosion, it goes to an almost steady state, Amable Liñán coined this mode as slowly reacting mode.
maximum temperature is unknown, but we have not used the boundary condition of the wall yet.
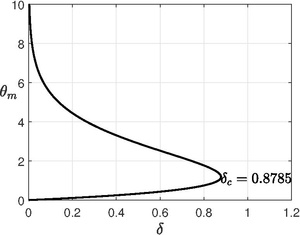
, the maximum temperature is obtained from an implicit expression, Critical
is obtained by finding the maximum point of the equation (see figure), i.e.,
are related to each other, which is obtained by substituting the above solution into the starting equation we arrive at
For spherical vessel, there is no known explicit solution, so Frank-Kamenetskii used numerical methods to find the critical value.
Unlike planar and cylindrical case, the spherical vessel has infinitely many solutions for
[20] The lowest branch will be chosen to explain explosive behavior.
From numerical solution, it is found that the critical Frank-Kamenetskii parameter is
For vessels which are not symmetric about the center (for example rectangular vessel), the problem involves solving a nonlinear partial differential equation instead of a nonlinear ordinary differential equation, which can be solved only through numerical methods in most cases.
Since the model assumes homogeneous mixture, the theory is well applicable to study the explosive behavior of solid fuels (spontaneous ignition of bio fuels, organic materials, garbage, etc.,).