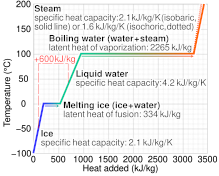
Specific heat capacity
If the amount is taken to be the volume of the sample (as is sometimes done in engineering), one gets the volumetric heat capacity, whose SI unit is joule per kelvin per cubic meter, J⋅m−3⋅K−1.
Black related an experiment conducted by Daniel Gabriel Fahrenheit on behalf of Dutch physician Herman Boerhaave.
Specific heat capacity is an intensive property of a substance, an intrinsic characteristic that does not depend on the size or shape of the amount in consideration.
[12]) The injection of heat energy into a substance, besides raising its temperature, usually causes an increase in its volume and/or its pressure, depending on how the sample is confined.
[13] The specific heat capacity can be defined and measured for gases, liquids, and solids of fairly general composition and molecular structure.
These include gas mixtures, solutions and alloys, or heterogenous materials such as milk, sand, granite, and concrete, if considered at a sufficiently large scale.
[14][15] The specific heat capacities of gases can be measured at constant volume, by enclosing the sample in a rigid container.
On the other hand, measuring the specific heat capacity at constant volume can be prohibitively difficult for liquids and solids, since one often would need impractical pressures in order to prevent the expansion that would be caused by even small increases in temperature.
Instead, the common practice is to measure the specific heat capacity at constant pressure (allowing the material to expand or contract as it wishes), determine separately the coefficient of thermal expansion and the compressibility of the material, and compute the specific heat capacity at constant volume from these data according to the laws of thermodynamics.
[19] Note the value's similarity to that of the calorie - 4187 J/kg⋅°C ≈ 4184 J/kg⋅°C (~.07%) - as they are essentially measuring the same energy, using water as a basis reference, scaled to their systems' respective lbs and °F, or kg and °C.
The temperature of a sample of a substance reflects the average kinetic energy of its constituent particles (atoms or molecules) relative to its center of mass.
Statistical mechanics predicts that at room temperature and ordinary pressures, an isolated atom in a gas cannot store any significant amount of energy except in the form of kinetic energy, unless multiple electronic states are accessible at room temperature (such is the case for atomic fluorine).
Therefore, the specific heat capacity (per gram, not per mole) of a monatomic gas will be inversely proportional to its (adimensional) atomic weight
For the noble gases, from helium to xenon, these computed values are On the other hand, a polyatomic gas molecule (consisting of two or more atoms bound together) can store heat energy in additional degrees of freedom.
Thus, the specific heat capacity per mole of a polyatomic gas depends both on the molecular mass and the number of degrees of freedom of the molecules.
[25][26][27] Quantum statistical mechanics predicts that each rotational or vibrational mode can only take or lose energy in certain discrete amounts (quanta), and that this affects the system’s thermodynamic properties.
Depending on the temperature, the average heat energy per molecule may be too small compared to the quanta needed to activate some of those degrees of freedom.
In that temperature range, the two additional degrees of freedom that correspond to vibrations of the atoms, stretching and compressing the bond, are still "frozen out".
The polytropic heat capacity is calculated at processes if all the thermodynamic properties (pressure, volume, temperature) change
The heat capacity must be zero at zero temperature in order for the above integral not to yield an infinite absolute entropy, thus violating the third law of thermodynamics.
For light and non-metallic elements, as well as most of the common molecular solids based on carbon compounds at standard ambient temperature, quantum effects may also play an important role, as they do in multi-atomic gases.
The path integral Monte Carlo method is a numerical approach for determining the values of heat capacity, based on quantum dynamical principles.
The International Union of Pure and Applied Chemistry (IUPAC) changed its recommendation from one atmosphere to the round value 100 kPa (≈750.062 Torr).
[notes 1] Measuring the specific heat capacity at constant volume can be prohibitively difficult for liquids and solids.
That is, small temperature changes typically require large pressures to maintain a liquid or solid at constant volume, implying that the containing vessel must be nearly rigid or at least very strong (see coefficient of thermal expansion and compressibility).
The polytropic heat capacity is calculated at processes if all the thermodynamic properties (pressure, volume, temperature) change:
In theory, the specific heat capacity of a substance can also be derived from its abstract thermodynamic modeling by an equation of state and an internal energy function.
To apply the theory, one considers the sample of the substance (solid, liquid, or gas) for which the specific heat capacity can be defined; in particular, that it has homogeneous composition and fixed mass
The absolute value of this quantity is undefined, and (for the purposes of thermodynamics) the state of "zero internal energy" can be chosen arbitrarily.
The path integral Monte Carlo method is a numerical approach for determining the values of heat capacity, based on quantum dynamical principles.