Persistent carbene
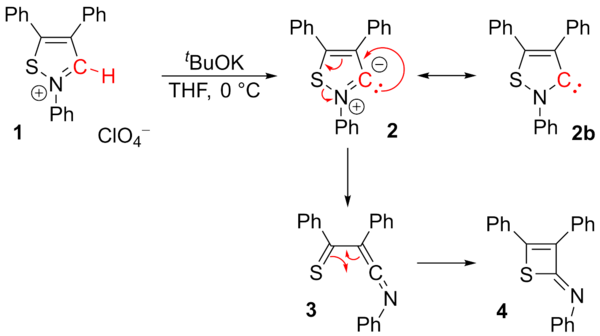
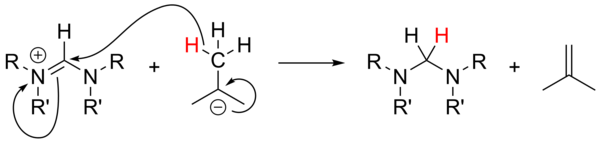
In 1957, Ronald Breslow proposed that a relatively stable nucleophilic carbene, a thiazol-2-ylidene derivative of vitamin B1 (thiamine), was the catalyst involved in the benzoin condensation that yields furoin from furfural.
[5][6] In 1960, Hans-Werner Wanzlick and coworkers conjectured that carbenes derived from dihydroimidazol-2-ylidene were produced by vacuum pyrolysis of the corresponding 2-trichloromethyl dihydroimidazole compounds with the loss of chloroform.
[14] Wanzlick as well as Roald Hoffmann,[9][15] proposed that these imidazole-based carbenes should be more stable than their 4,5-dihydro analogues, due to Hückel-type aromaticity.
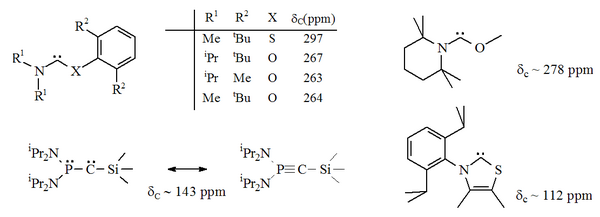
These species can be represented as either a λ3-phosphinocarbene or λ5-phosphaacetylene:[16][17] These compounds were called "push-pull carbenes" in reference to the contrasting electron affinities of the phosphorus and silicon atoms.
An X-ray structure of this molecule has not been obtained and at the time of publication some doubt remained as to their exact carbenic nature.
[23][24] In the modern understanding, the superficially unoccupied p-orbital on a (meta)stable carbene is not, in fact, fully empty.
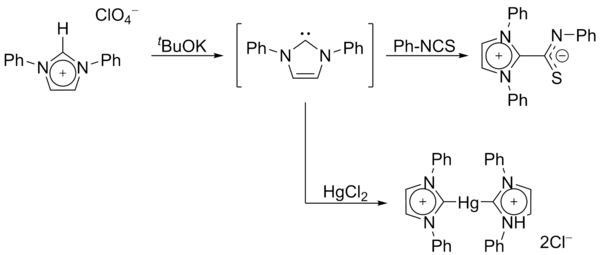
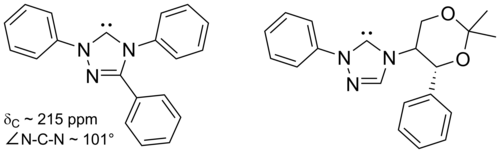
[26] In 1995, Arduengo's group obtained a carbene derivative of dihydroimidazol-2-ylidene, proving that stability did not arise from the aromaticity of the conjugated imidazole backbone.
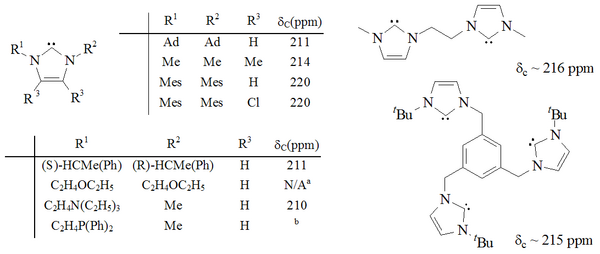
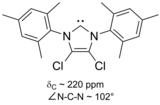
[citation needed] A considerable range of imidazol-2-ylidenes have been synthesised, including those in which the 1,3-positions have been functionalised with alkyl, aryl,[26] alkyloxy, alkylamino, alkylphosphino[36] and even chiral substituents:[36] In particular, substitution of two chlorine atoms for the two hydrogens at ring positions 4 and 5 yielded the first air-stable carbene.
[21] Its extra stability probably results from the electron-withdrawing effect of the chlorine substituents, which reduce the electron density on the carbon atom bearing the lone pair, via induction through the sigma-backbone.
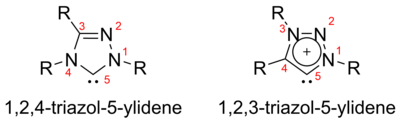
[28][31][32] Unlike the aromatic imidazol-2-ylidenes or triazol-5-ylidenes, these carbenes appear not to be thermodynamically stable, as shown by the dimerisation of some unhindered cyclic and acyclic examples.
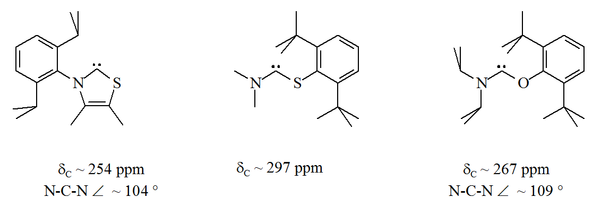
[34] Since oxygen and sulfur are divalent, steric protection of the carbenic centre is limited especially when the N–C–X unit is part of a ring.
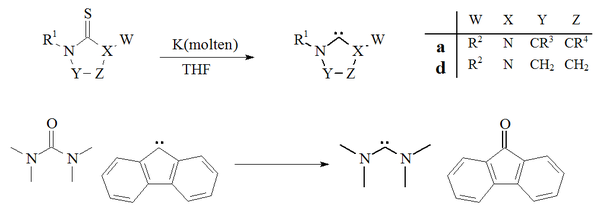
[citation needed] Carbenes that formally derive from imidazole-2-ylidenes by substitution of sulfur, oxygen, or other chalcogens for both α-nitrogens are expected to be unstable, as they have the potential to dissociate into an alkyne (R1C≡CR2) and a carbon dichalcogenide (X1=C=X2).
[44] However, these compounds seem to exhibit some alkynic properties, and when published the exact carbenic nature of these red oils was in debate.
[17] One stable N-heterocyclic carbene[45] has a structure analogous to borazine with one boron atom replaced by a methylene group.
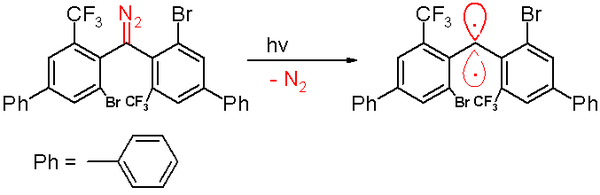
[48] This carbene is prepared by a photochemical decomposition of a diazomethane precursor by 300 nm light in benzene with expulsion of nitrogen gas.
Again the figure below is not an adequate representation of the actual molecular structure: both phenyl rings are positioned orthogonal with respect to each other.
Based on computer simulations, the distance of the divalent carbon atom to its neighbors is claimed to be 138 picometers with a bond angle of 158.8°.
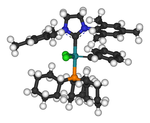
A periodic table of elements gives some idea of the complexes which have been prepared, and in many cases these have been identified by single crystal X-ray crystallography.
[40][59][60] Stable carbenes are believed to behave in a similar fashion to organophosphines in their coordination properties to metals.
Several catalytic systems have been looked into by Hermann and Enders, using catalysts containing imidazole and triazole carbene ligands, with moderate success.
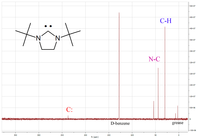
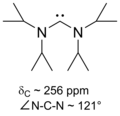
Upon coordination to metal centers, the 13C carbene resonance usually shifts highfield, depending on the Lewis acidity of the complex fragment.
Based on this observation, Huynh et al. developed a new methodology to determine ligand donor strengths by 13C NMR analysis of trans-palladium(II)-carbene complexes.
The use of a 13C-labeled N-heterocyclic carbene ligand also allows for the study of mixed carbene-phosphine complexes, which undergo trans-cis-isomerization due to the trans effect.
[70] In these cases, strong unhindered nucleophiles are avoided whether they are generated in situ or are present as an impurity in other reagents (such as LiOH in BuLi).
However, these catalysts have proved ineffective for the preparation of non-imidazolium adducts as they tend to act as nucleophiles towards the precursor salts and in so doing are destroyed.
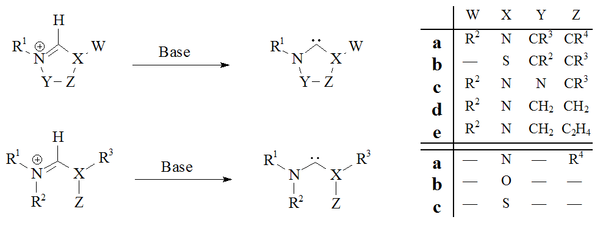
[29] Vacuum pyrolysis, with the removal of neutral volatile byproducts i.e. methanol or chloroform, has been used to prepare dihydroimidazole and triazole based carbenes.
Historically the removal of chloroform by vacuum pyrolysis of adducts A was used by Wanzlick[8] in his early attempts to prepare dihydroimidazol-2-ylidenes but this method is not widely used.
[73] For example: Persistent triplet state carbenes have been prepared by photochemical decomposition of a diazomethane product via the expulsion of nitrogen gas, at a wavelength of 300 nm in benzene.
However, provided rigorously dry, relatively non-acidic and air-free materials are used, stable carbenes are reasonably robust to handling per se.
By way of example, a stable carbene prepared from potassium hydride can be filtered through a dry celite pad to remove excess KH (and resulting salts) from the reaction.





( View the 3D structure with external viewer. )







| a | 3,6-diphenyl-1,2,4,5-tetrazine, toluene | 92% | e | 2 equiv., PhNCO, toluene, reflux | 92% | |
|---|---|---|---|---|---|---|
| b | RXH, RT | 95–97% | f | CS 2 , toluene, or PhNCS, THF, RT | 71–90% | |
| c | O 2 , S 8 , or Se, toluene, reflux | 54–68% | g | Maleimide, THF, RT | 47–84% | |
| d | R 1 CH=CHR 2 , THF, RT | 25–68% | h | Dimethylacetylene dicarboxylate, THF, reflux | 21% |