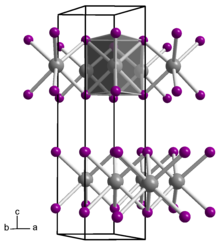
Gadolinium diiodide
Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2.
It is an electride, with the ionic formula of Gd3+(I−)2e−,[2] and therefore not a true gadolinium(II) compound.
It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance (~70%) at 7 T near room temperature.
[3] It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature:[4] It can react with hydrogen at high temperature (800 °C) to obtain gadolinium hydrogen iodide (GdI2H0.97[5]).
[6] This inorganic compound–related article is a stub.