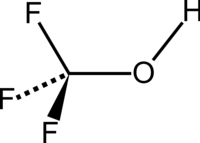
Trifluoromethanol
This fact can be explained by the absence of intramolecular H—F bonds, which are also not visible in the infrared gas phase spectrum.
A simpler synthesis uses the reaction (I); an equilibrium can be shifted to the thermodynamically preferred trifluoromethanol at lower temperatures.
If the synthesized trifluoromethanol is protonated by superacids, for example HSbF6 (fluoroantimonic acid), the equilibrium can be further shifted to the left towards the desired product.
However, if the CF3O− ion is, for example, in an aqueous solution displaced by an acid, trifluoromethanol decomposes at the room temperature.
In this case, decomposition of trifluoromethanol is negligible under the conditions prevailing in the atmosphere due to the high activation energy of the reaction.