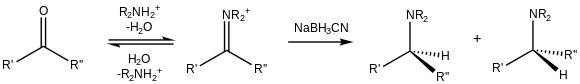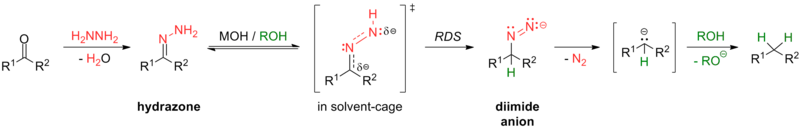Carbonyl reduction
In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H. Two broad strategies exist for carbonyl reduction.
For example, zinc borohydride, nominally Zn(BH4)2, is used for mild selective reduction of aldehydes and ketones in the presence of other reducible groups.
[6] In their handling properties, lithium aluminium hydride and sodium borohydride (and their derivatives) strongly differ.
Substituents on the boron or aluminium modulate the power, selectivity, and handling properties of these reducing agents.
[10] In the Fukuyama reduction, a carboxylic acid is first converted to a thioester through addition of a thiol (with a mechanism similar to esterification).
DIBAL-H can selectively reduce acid chlorides to the aldehyde level if only one equivalent is used at low temperatures.
[13] The idealized equation for the reduction of an acid chloride to an aldehyde by lithium aluminium hydride is: The traditional method of forming aldehydes without reducing to alcohols - by using hindered hydrides and reactive carbonyls - is limited by its narrow substrate scope and great dependence on reaction conditions.
In the Weinreb ketone synthesis, an acyl chloride is first converted to the Weinreb amide, then treated with an organometallic reagent to form a ketone, or lithium aluminum hydride to form an aldehyde:[14] The Weinreb amide is reduced via a stable chelate, rather than the electrophilic carbonyl that is formed through metal hydride reductions; the chelate is therefore only reduced once, as illustrated below: The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
[15] Ketones are less reactive than aldehydes, because of greater steric effects, and because the extra alkyl group contributes electron density to the C=O bond, making it less electrophilic.
At the other extreme, carboxylic acids, amides, and esters are poorly electrophilic and require strong reducing agents.
[18] Because of its cyano substituent, NaBH3CN is a weak reducer at moderate pH (>4), so it preferentially reduces iminium cations that exist in the presence of carbonyls: When an α,β-unsaturated carbonyl is reduced, three products can result: an allyl alcohol from simple carbonyl reduction, a saturated ketone or aldehyde resulting from 1,4‑reduction (also called conjugate reduction), or the saturated alcohol from double reduction.
[20] Triphenylphosphinocopper hydride clusters directs catalytic hydrogenation to perform specifically conjugate reduction.
[26] For example, ketones are reduced to their respective alkyl benzenes by catalytic hydrogenation[27][28] or by Birch reduction[29] under mild conditions.
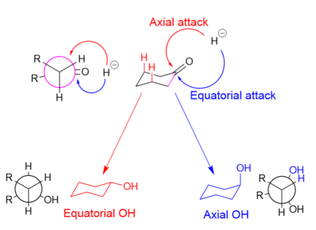
In equatorial attack (shown in blue), the hydride avoids the 1,3-diaxial interaction, but the substrate undergoes unfavorable torsional strain when the newly formed alcohol and added hydrogen atom eclipse each other in the reaction intermediate (as shown in the Newman projection for the axial alcohol).
[30] Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
[33] Salts boron and aluminium hydrides, discovered starting in the 1940s, proved to be highly convenient reagents for carbonyl reduction.