H3K56ac
It is a covalent modification known as a mark of newly replicated chromatin as well as replication-independent histone replacement.
Lysine 56 is located at the amino-terminal αN-helix and close to the site where the DNA enters and exits the nucleosome.
Sirtuins can catalyze the removal of the acetyl group from K56[1] H3K56ac levels are elevated in cancer and pluripotent cells.TRIM66 reads unmodified H3R2K4 and H3K56ac to respond to DNA damage.
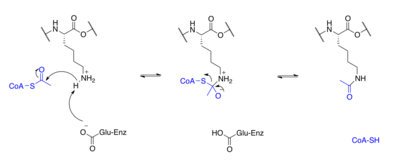
[2] The regulation of transcription factors, effector proteins, molecular chaperones, and cytoskeletal proteins by acetylation and deacetylation is a significant post-translational regulatory mechanism[3] These regulatory mechanisms are analogous to phosphorylation and dephosphorylation by the action of kinases and phosphatases.
Not only can the acetylation state of a protein modify its activity but there has been recent suggestion that this post-translational modification may also crosstalk with phosphorylation, methylation, ubiquitination, sumoylation, and others for dynamic control of cellular signaling.
[4][5][6] In the field of epigenetics, histone acetylation (and deacetylation) have been shown to be important mechanisms in the regulation of gene transcription.
[10] The current understanding and interpretation of histones comes from two large scale projects: ENCODE and the Epigenomic roadmap.
This led to chromatin states which define genomic regions by grouping the interactions of different proteins and/or histone modifications together.
[16] H3K56ac is important for chromatin remodeling and serves as a marker of new nucleosomes during DNA replication but its role in the cell cycle is debated.
[1] Lysine 56 is located at the amino-terminal αN-helix and close to the site where the DNA enters and exits the nucleosome.
[1] Sirtuins can catalyze the removal of the acetyl group from K56[1] H3K56ac levels are elevated in cancer and pluripotent cells[1] TRIM66 reads unmodified H3R2K4 and H3K56ac to respond to DNA damage.