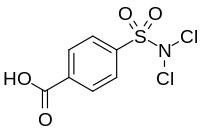
Halazone
Halazone (4-(dichlorosulfamoyl)benzoic acid) is a chemical compound whose formula can be written as either C7H5Cl2NO4S or (HOOC)(C6H4)(SO2)(NCl2).
[5][6] Halazone tablets were commonly used during World War II by U.S. soldiers for portable water purification, even being included in accessory packs for C-rations until 1945.
[citation needed] Dilute halazone solutions (4 to 8 ppm of available chlorine) has also been used to disinfect contact lenses,[8] and as a spermicide.
Halazone's disinfecting activity is mainly due to the hypochlorous acid (HClO) released by hydrolysis of the chlorine-nitrogen bonds when the product is dissolved in water:[8] The hypochlorous acid is a powerful oxidizer and chlorinating agent that destroys or denatures many organic compounds.
[4] Another synthesis route is the oxidation of dichloramine-T with potassium permanganate in a mild alkaline medium.