Half-reaction
For oxidation-reduction reactions in basic conditions, after balancing the atoms and oxidation numbers, first treat it as an acidic solution and then add OH− ions to balance the H+ ions in the half reactions (which would give H2O).
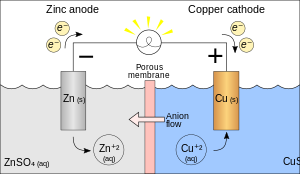
Consider the Galvanic cell shown in the adjacent image: it is constructed with a piece of zinc (Zn) submerged in a solution of zinc sulfate (ZnSO4) and a piece of copper (Cu) submerged in a solution of copper(II) sulfate (CuSO4).
The overall reaction is: At the Zn anode, oxidation takes place (the metal loses electrons).
This is represented in the following reduction half reaction (note that the electrons are on the reactants side): Consider the example burning of magnesium ribbon (Mg).
After canceling, the equation is re-written as Two ions, positive (Mg2+) and negative (O2−) exist on product side and they combine immediately to form a compound magnesium oxide (MgO) due to their opposite charges (electrostatic attraction).
Due to this electrolyte it may be more difficult to satisfy the balance of both the atoms and charges.
Often there will be both H+ and OH− present in acidic and basic conditions but that the resulting reaction of the two ions will yield water, H2O (shown below):