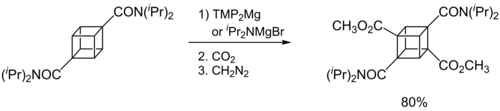Hauser base
[1] Compared with organolithium reagents, the magnesium compounds have more covalent, and therefore less reactive, metal-ligand bonds.
Consequently, they display a higher degree of functional group tolerance and a much greater chemoselectivity.
[4][6][7] The structures of Hauser bases in solution have been investigated by diffusion-ordered NMR spectroscopy (DOSY).
In consequence, the metalation rates are slow and a large excess of base is required (e.g., 10 equiv.).
Improved solubility and reactivity can be achieved by adding stoichiometric amounts of LiCl to the Hauser base.