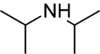
Diisopropylamine
[5] It reacts with organolithium reagents to give lithium diisopropylamide (LDA).
LDA is a strong, non-nucleophilic base[6] The main commercial applications of diisopropylamine is as a precursor to the herbicide, diallate and triallate as well as certain sulfenamides used in the vulcanization of rubber.
[7] It is also used to prepare N,N-diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate.
[9] Diisopropylamine, which is commercially available, may be prepared by the reductive amination of acetone with ammonia using a modified copper oxide, generally copper chromite, as a catalyst:[10][11] Diisopropylamine can be dried by distillation from potassium hydroxide (KOH) or drying over sodium wire.
Inhalation of high concentrations of its vapor may cause symptoms like headache, dizziness, tiredness, nausea and vomiting.