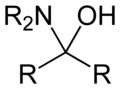
Hemiaminal
Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution.
[2] The adducts formed by the addition of ammonia to aldehydes have long been studied.
[3] Compounds containing both a primary amino group and a hydroxyl group bonded to the same carbon atom are rarely stable, as they tend to dehydrate to form imines which polymerise to hexamethylenetetramine.
This reaction produces first the carbinolamine (a hemiaminal) and bis(dimethylamino)methane (Me = CH3):[7][8] The reaction of formaldehyde with carbazole, which is weakly basic, proceed similarly:[9] Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Hemiaminal formation is a key step in an asymmetric total synthesis of saxitoxin:[10] In this reaction step the alkene group is first oxidized to an intermediate acyloin by action of osmium(III) chloride, oxone (sacrificial catalyst) and sodium carbonate (base).