Hemorheology
Blood becomes less viscous at high shear rates like those experienced with increased flow such as during exercise or in peak-systole.
This biophysical property makes it a critical determinant of friction against the vessel walls, the rate of venous return, the work required for the heart to pump blood, and how much oxygen is transported to tissues and organs.
These functions of the cardiovascular system are directly related to vascular resistance, preload, afterload, and perfusion, respectively.
[7] Furthermore, elevation of plasma viscosity correlates to the progression of coronary and peripheral artery diseases.
of blood at 37 °C is normally 3 × 10−3 to 4 × 10−3,[8] respectively 3 - 4 centipoise (cP) in the centimetre gram second system of units.
Calculations have shown that the maximum volume percentage of red blood cells without deformation is 58% which is in the range of normally occurring levels.
This interaction and tendency for cells to aggregate is a major contributor to the viscoelastic behavior of blood.
Red blood cell deformation and aggregation is also coupled with flow-induced changes in the arrangement and orientation as a third major factor in its viscoelastic behavior.
[13] When the red cells are at rest or at very small shear rates, they tend to aggregate and stack together in an energetically favorable manner.
With very low shear rates, the viscoelastic property of blood is dominated by the aggregation and cell deformability is relatively insignificant.
As shear rates become large, red blood cells will stretch or deform and align with the flow.
Complicating moreover blood volume shape, red cells are not identically distributed in a blood sample volume because they migrate with velocity gradients in direction to the highest speed areas calling the famous representation of the Fåhræus–Lindqvist effect, aggregate or separate in sheath or plug flows described by Thurston.
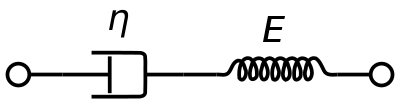
[15] Typically, the Maxwell Model described below is uniformly considering the material (uniform blue color) as a perfect distributed particles fluid everywhere in the volume (in blue) but Thurston reveals that packs of red cells, plugs, are more present in the high speed region, if y is the height direction in the Maxwell model figure, (y~H) and there is a free cells layer in the lower speed area (y~0) what means the plasma fluid phase that deforms under Maxwell Model is strained following inner linings that completely escape from the analytical model by Maxwell.
[citation needed] In theory, a fluid in a Maxwell Model behaves exactly similarly in any other flow geometry like pipes, rotating cells or in rest state.
In steady flow, the slippage will continue to increase and the measurements of non time varying force will neglect the contributions of the elasticity.
Therefore, the size and phase relation between the stress, strain, and shear rate are described using this relationship and a radian frequency,
Here we consider a three-dimensional Oldroyd-B model coupled with the momentum equation and the total stress tensor.
There are several methods used to explore the mechanical properties of red blood cells such as: These methods worked to characterize the deformability of the red blood cell in terms of the shear, bending, area expansion moduli, and relaxation times.
[21] Another experimental technique used to evaluate viscoelasticity consisted of using Ferromagnetism beads bonded to a cells surface.
The hysteresis shown in figure 3 represents the viscoelasticity present in red blood cells.
It is unclear if this is related to membrane molecular fluctuations or metabolic activity controlled by intracellular concentrations of ATP.
Further research is needed to fully explore these interaction and to shed light on the underlying viscoelastic deformation characteristics of the red blood cells.
When looking at viscoelastic behavior of blood in vivo, it is necessary to also consider the effects of arteries, capillaries, and veins.
[26] This has also led the way for developing a blood analog in order to study and test prosthetic devices.
The classic analog of glycerin and water provides a good representation of viscosity and inertial effects but lacks the elastic properties of real blood.
[29] Professor George B. Thurston, of the University of Texas, first presented the idea of blood being viscoelastic in 1972.
[30][31] Advancements in medical procedures and devices required a better understanding of the mechanical properties of blood.
Given the complex macro-rheological behavior of blood, it is not surprising that a single equation fails to completely describe the effects of various rheological variables (e.g., hematocrit, shear rate).
Thus, several approaches to defining these equations exist, with some the result of curve-fitting experimental data and others based on a particular rheological model.
Often, this limit of the applicability of the continuum model begins to manifest itself at characteristic channel dimensions that are about 30 times the particle diameter: in the case of blood with a characteristic RBC dimension of 8 μm, an apparent failure occurs at about 300 micrometres.