Hexachlorophosphazene
The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen atoms, and can be viewed as a trimer of the hypothetical compound N≡PCl2 (phosphazyl dichloride).
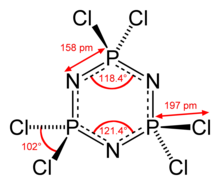
[2][5] Cyclophosphazenes such as hexachlorophosphazene are distinguished by notable stability and equal P–N bond lengths which, in many such cyclic molecules, would imply delocalization or even aromaticity.
To account for these features, early bonding models starting from the mid-1950s invoked a delocalised π system arising from the overlap of N 2p and P 3d orbitals.
[2][3] Starting from the late 1980s, more modern calculations and the lack of spectroscopic evidence reveal that the P 3d contribution is negligible, invalidating the earlier hypothesis.
[1][3] The rest (~15%) of the bond strength may be attributed to a negative hyperconjugation interaction: the N lone pairs can donate some electron density into π-accepting σ* molecular orbitals on the P.[3] The synthesis of hexachlorophosphazene was first reported by von Liebig in 1834.
[9] They found that phosphorus pentachloride (PCl5) and ammonia (NH3) react exothermically to yield a new substance that could be washed with cold water to remove the ammonium chloride ([NH4]Cl) coproduct.
[2] Modern syntheses are based on the developments by Schenk and Römer who used ammonium chloride in place of ammonia and inert chlorinated solvents.
[6] Reaction conditions such as temperature may also be tuned to maximise the yield of the trimer at the expense of the other possible products; nonetheless, commercial samples of hexachlorophosphazene usually contain appreciable amounts of octachlorotetraphosphazene, even up to 40%.
[2][4][6] The nitrogen centres of hexachlorophosphazene are weakly basic, and this Lewis base behaviour has been suggested to play a role in the polymerisation mechanism.
[7] Hexachlorophosphazene has also found applications in research by enabling aromatic coupling reactions between pyridine and either N,N-dialkylanilines or indole, resulting in 4,4'-substituted phenylpyridine derivatives, postulated to go through a cyclophosphazene pyridinium salt intermediate.
[6] The hexalkoxyphosphazenes (especially the aryloxy species), resulting from the nucleophilic hexasubstitution of the hexachlorophosphazene P atoms, have attracted interest for their high thermal and chemical stability as well as their low glass transition temperature.
[2][4] Some of them appear promising for future applications as fibre- or membrane-forming high performance materials, since they combine transparency, backbone flexibility, tunable hydrophilicity or hydrophobicity, and various other desirable properties.
[4] Polyphosphazene-based components have been used in O-rings, fuel lines and shock absorbers, where the polyphosphazenes confer fire resistance, imperviousness to oils, and flexibility even at very low temperatures.