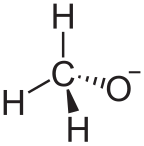
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom.
Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis.
The metal hydride removes the hydrogen atom from the hydroxyl group and forms a negatively charged alkoxide ion.
Oxo-ligands typically arise via the hydrolysis, often accidentally, and via ether elimination:[citation needed] Many metal alkoxides thermally decompose in the range ≈100–300 °C.
This method represents a prospective approach possessing an advantage of capability of obtaining functional materials with increased phase and chemical homogeneity and controllable grain size (including the preparation of nanosized materials) at relatively low temperature (less than 500–900 °C) as compared with the conventional techniques.
[6] It is used as an initiator of an anionic addition polymerization with ethylene oxide, forming a polyether with high molecular weight.
[citation needed] Both sodium methoxide and its counterpart prepared with potassium are frequently used as catalysts for commercial-scale production of biodiesel.