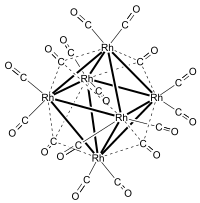
Hexadecacarbonylhexarhodium
Rh6(CO)16 was first prepared by Hieber in 1943 by carbonylation of RhCl3·3H2O at 80–230 °C and 200 atm carbon monoxide with silver or copper as a halide acceptor.
Hieber correctly formulated the compound as a binary carbonyl, but suggested the formula Rh4(CO)11, i.e., CO/Rh ratio of 2.75.
[2] The correct formula and structure was subsequently established by Dahl et al. using X-ray crystallography.
[3] Relative to the original preparation, the carbonylation of a mixture of anhydrous rhodium trichloride and iron pentacarbonyl was shown to give good yields of Rh6(CO)16.
[4] Other compounds of rhodium are also effective precursors such as [(CO)2Rh(μ-Cl)]2 and rhodium(II) acetate:[1] It also arises quantitatively by thermal decomposition of tetrarhodium dodecacarbonyl in boiling hexane:[5] At least some of the CO ligands can be displaced by donor ligands.