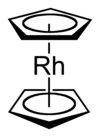
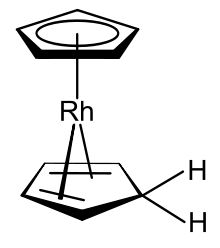
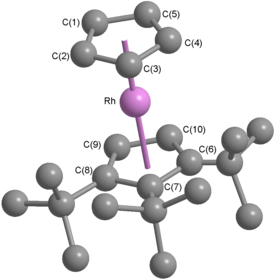
Rhodocene
[11] The study of organometallic species including these ultimately led to the development of new bonding models that explained their formation and stability.
[12][13] Work on sandwich compounds, including the rhodocenium-rhodocene system, earned Geoffrey Wilkinson and Ernst Otto Fischer the 1973 Nobel Prize for Chemistry.
The original synthesis used a cyclopentadienyl anion and tris(acetylacetonato)rhodium(III);[11] numerous other approaches have since been reported, including gas-phase redox transmetalation[16] and using half-sandwich precursors.
[17] Octaphenylrhodocene (a derivative with eight phenyl groups attached) was the first substituted rhodocene to be isolated at room temperature, though it decomposes rapidly in air.
[28] Ferrocene, [Fe(C5H5)2], was first synthesised in 1951 during an attempt to prepare the fulvalene (C10H8) by oxidative dimerization of cyclopentadiene; the resultant product was found to have molecular formula C10H10Fe and reported to exhibit "remarkable stability".
[10] The discovery sparked substantial interest in the field of organometallic chemistry,[8][9] in part because the structure proposed by Pauson and Kealy was inconsistent with then-existing bonding models and did not explain its unexpected stability.
[32] Applying valence bond theory to ferrocene by considering an Fe2+ centre and two cyclopentadienide anions (C5H5−), which are known to be aromatic according to Hückel's rule and hence highly stable, allowed correct prediction of the geometry of the molecule.
[13] The properties of cobaltocene reported by Wilkinson and Fischer demonstrated that the unipositive cobalticinium cation [Co(C5H5)2]+ exhibited stability similar to that of ferrocene itself.
In the same study, attempts to detect iridocene by exposing iridocenium salts to oxidising conditions were unsuccessful even at elevated pH.
"[34] This helps to explain the unusually high stability observed for ferrocene[10] and for the cobalticinium and rhodocenium cations[31] – all three species have analogous geometries and are isoelectronic 18-valence electron structures.
[34] Rhodocene exists as [Rh(C5H5)2], a paramagnetic 19-valence electron radical monomer only at or below −196 °C (−320.8 °F) (liquid nitrogen temperatures) or above 150 °C (302 °F) in the gas phase.
[4] ESR evidence confirms that the monomer possesses a high order axis of symmetry (Cn, n > 2) with a mirror plane (σ) perpendicular to it as symmetry elements; this experimentally demonstrates that the monomer does possess the typical sandwich structure of a metallocene[3][Note 2] although the interpretation of the ESR data has been questioned.
The term hapticity is used to indicate the "number of carbon (or other) atoms through which [a ligand] binds (n)"[38] to a metal centre and is symbolised as ηn.
For example, the ethylene ligand in Zeise's salt is bound to the platinum centre through both carbon atoms, and it hence formally has the formula K[PtCl3(η2-C2H4)]·H2O.
Fischer and colleagues hypothesised that the formation of this rhodocene derivative might occur in separate protonation and reduction steps, but published no evidence to support this suggestion.
It has been shown that this compound can also be prepared by sodium borohydride reduction of a rhodocenium solution in aqueous ethanol; the researchers who made this discovery characterised the product as biscyclopentadienylrhodium hydride.
[3][4] The complex [(η5-C5H5)Ir(η4-C5H6)], the analogue of rhodocene derivative reported by Fischer,[3] has also been studied and demonstrates properties consistent with a greater degree of π-backbonding in iridium(I) systems than is found in the analogous cobalt(I) or rhodium(I) cases.
[10] These salts were prepared by reacting the carbanion Grignard reagent cyclopentadienylmagnesium bromide (C5H5MgBr) with tris(acetylacetonato)rhodium(III) (Rh(acac)3).
[3] If a rhodocenium containing melt is treated with sodium or potassium metals and then sublimed onto a liquid nitrogen-cooled cold finger, a black polycrystalline material results.
The crystal structure of the hexafluorophosphate salt shows three crystallographically independent cations, one eclipsed, one staggered, and one which is rotationally disordered.
[48] The following data are presented relative to the ferrocenium / ferrocene redox couple:[49] The differences in reduction potentials are attributed in the cobaltocenium system to the inductive effect of the alkyl groups,[19] further stabilising the 18-valence electron species.
[48] The irreversibility of the substituted iridocenium reductions is attributed to the extremely rapid dimerisation of the resulting 19-valence electron species, which further illustrates that iridocenes are less stable than their corresponding rhodocenes.
[51] Substitutions on the cyclopentadienyl rings of rhodocenes and rhodocenium salts produce compounds of higher stability as they allow for the increased delocalisation of positive charge or electron density and also provide steric hindrance against other species approaching the metal centre.
[18][Note 5] Decamethylrhodocenium tetrafluoroborate can be synthesised from the tris(acetone) complex [(η5-C5Me5)Rh(Me2CO)3](BF4)2 by reaction with pentamethylcyclopentadiene, and the analogous iridium synthesis is also known.
[53] These reactions demonstrate that the acidity of the methyl hydrogens in a pentamethylcyclopentadienyl complex can be considerably increased by the presence of the metal centre.
[18] Cobaltocene is a useful one-electron reducing agent in the research laboratory as it is soluble in non-polar organic solvents,[19] and its redox couple is sufficiently well behaved that it may be used as an internal standard in cyclic voltammetry.
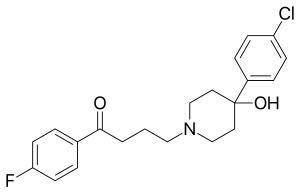
One area of such research has utilised metallocenes in place of the fluorophenyl group in haloperidol,[21] which is a pharmaceutical classified as a typical antipsychotic.
[20] The original motivation for research investigations of the rhodocene system was to understand the nature of and bonding within the metallocene class of compounds.
In more recent times, interest has been rekindled by the desire to explore and apply the metal–metal interactions that occur when metallocene systems are linked.
[61] Taking as an example the 1-cobaltocenyl-1'-rhodocenylferrocene cation shown above, this means that the cobaltocenyl and rhodocenyl moieties are eclipsed, and thus carbon atoms 1 and 1' on the central ferrocene core are as close to vertically aligned as is possible given the staggered conformation of the cyclopentadienyl rings within each metallocene unit.