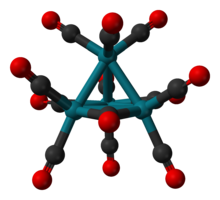
Tetrarhodium dodecacarbonyl
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12.
[1] Rh4(CO)12 is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper metal under an atmosphere of CO.[2] Alternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H[RhCl2(CO)2], followed by carbonylation in the presence of sodium citrate.
[1] The cluster undergoes thermal substitution with phosphine ligands, L:[3] Tetrarhodium dodecacarbonyl quantitatively decomposes in boiling hexane to afford hexadecacarbonylhexarhodium:[4] Because of their relevance to hydroformylation catalysis, rhodium carbonyls have been systematically studied to a high degree.
Solutions of Rh4(CO)12 under high pressures of CO convert to the dirhodium compound:[5] Unlike Co2(CO)8 which features bridging carbonyls, the main isomer of Rh2(CO)8 features only terminal CO ligands.
The relative instability of Rh2(CO)8 is analogous to the tendency of Ru(CO)5 to convert to Ru3(CO)12.