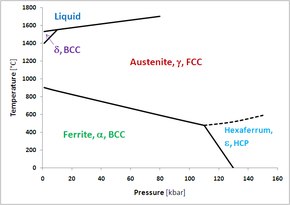
Hexaferrum
A 1964 study at the University of Rochester mixed 99.8% pure α-iron powder with sodium chloride, and pressed a 0.5-mm diameter pellet between the flat faces of two diamond anvils.
The deformation of the NaCl lattice, as measured by x-ray diffraction (XRD), served as a pressure indicator.
At a pressure of 13 GPa and room temperature, the body-centered cubic (BCC) ferrite powder transformed to the HCP phase in Figure 1.
An extrapolation of the austenite-hexaferrum phase boundary in Figure 1 suggests hexaferrum could be stable or metastable in Earth's core.
[1] For this reason, many experimental studies have investigated the properties of HCP iron under extreme pressures and temperatures.