Antimony pentafluoride
inhalation, 2 hours) Antimony pentafluoride is the inorganic compound with the formula SbF5.
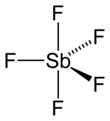
[6] In the gas phase, SbF5 adopts a trigonal bipyramidal structure of D3h point group symmetry (see picture).
[7] The related species PF5 and AsF5 are monomeric in the solid and liquid states, probably due to the smaller sizes of the central atom, which limits their coordination number.
SbF5 is a strong Lewis acid, exceptionally so toward sources of F− to give the very stable anion [SbF6]−, called hexafluoroantimonate.
Although it is only weakly basic, [SbF6]− does react with additional SbF5 to give a centrosymmetric adduct: The [Sb2F11]− anion is one of the ions found in HF/SbF5 Mixture.