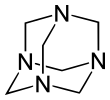
Hexamethylenetetramine
[2][3] It has the chemical formula (CH2)6N4 and is a white crystalline compound that is highly soluble in water and polar organic solvents.
[6][7] It is prepared industrially by combining formaldehyde and ammonia:[8] The molecule behaves like an amine base, undergoing protonation and as a ligand.
These products are used as binders, e.g., in brake and clutch linings, abrasives, non-woven textiles, formed parts produced by moulding processes, and fireproof materials.
[4][10][11][12] It is used as an alternative to antibiotics to prevent urinary tract infections (UTIs) and is sold under the brand names Hiprex, Urex, and Urotropin, among others.
[13][14] A systematic review of its use for this purpose in adult women found there was insufficient evidence of benefit and further research was needed.
[citation needed] Standardized 0.149 g tablets of methenamine (hexamine) are used by fire-protection laboratories as a clean and reproducible fire source to test the flammability of carpets and rugs.
From October 2023, sale of hexamethylenetetramine in the UK is restricted to licenced persons (as a "regulated precursor" under the terms of the Poisons Act 1972).
[28] Because of its ash-free combustion, hexamethylenetetramine is also utilized in indoor fireworks alongside magnesium and lithium salts.
[33] Scientist De Eds found that there was a direct correlation between the acidity of hexamethylenetetramine's environment and the rate of its decomposition.