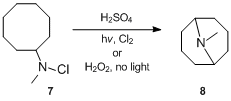
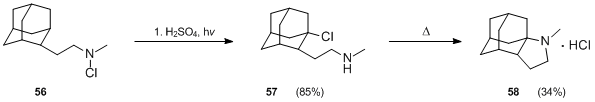
Hofmann–Löffler reaction
[11][12][13] Noting that the hydrogen peroxide or ultraviolet light greatly improved yields, Wawzonek and Thelan[11] suggested a free-radical mechanism.
According to Wawzonek and Thelan's 1949 proposal,[11] an acid first protonates an N-chloroamine, which, in the presence of heat, light, or other initiators, homolyzes to ammonium and chloride free radicals.
Comparable reactions at a primary carbon also give kH⁄kD≫1, which strongly suggests that the breaking of the C-H bond proceeds to a rather considerable extent in the transition state.
[14] An important question in discussing the role of the acid is whether the N-haloamine reacts in the free base or the salt form in the initiation step.
As a result, in the case of chemical or thermal initiation, it is reasonable to assume that it is the N-chloroammonium ion which affords the ammonium free radical.
The radiation must be absorbed and the quantum of the incident light must be large enough to dissociate the N-Cl bond in order for a photochemical reaction to occur.
However, it is important to realize that an alternative scenario might be in operation when the reaction is initiated with the UV light; namely, the free N-haloamine might not undergo dissociation upon irradiation, but it might function as a photosensitizer instead.
In order to determine the structural and geometrical factors affecting the intramolecular hydrogen atom transfer, a number of different N-chloroamines were examined in the Hofmann–Löffler–Freytag reaction.
In terms of the geometrical requirements in the intramolecular rearrangement of hydrogen, it was observed that under identical reaction conditions the UV light-catalyzed decomposition of methylcyclohexylchloroamine and N-chloroazacycloheptane proceeds far more slowly than that of dibutylchloroamine.
These findings indicate that the prevailing geometries are in these two cases unfavourable for the rearrangement to occur and the Cδ–H–N bond angle required for the intramolecular hydrogen transfer cannot be easily attained.
In contrast, it has been argued that the UV light-catalyzed initiation involves the free form of the N-haloamine and a rapid protonation of the newly generated neutral nitrogen radical (see the section devoted to mechanistic studies for arguments supporting this statement).
[19] The great advantage of the Suárez modification is that the reaction can be performed under very mild neutral conditions compatible with the stability of the protective groups most frequently used in synthetic organic chemistry.
P. E. Sonnet and J. E. Oliver[32] employed classic Hofmann–Löffler–Freytag reaction conditions in the synthesis of potential ant sex pheromone precursors (i.e. octahydroindolizine 51).
[34] Irradiation of 54 with a 400 W high-pressure mercury lamp in trifluoroacetic acid under a nitrogen atmosphere at room temperature for 5 h afforded a moderate yield of the product.
In case of 64 and 66, the five-membered nitrogen ring is formed by attack on the unactivated C-18 methyl group of the precursor (63 or 65, respectively) by a suitably placed nitrogen-centered radical at C-20.
The ease of this reaction is due to the fact that in the rigid steroid framework the β-C-18 methyl group and the β-C-20 side chain carrying the nitrogen radical are suitably arranged in space in order to allow the 1,5-hydrogen abstraction to proceed via the six-membered transition state.
A very interesting transformation is observed when sulfonamides of primary amides bearing an aromatic ring at the γ-position are treated with various iodanes and iodine under the irradiation with a tungsten lamp.
[45] E. Suárez et al.[46] reported that the amidyl radical intermediates, produced by photolysis of medium-sized lactams, e.g. 82 in the presence of PhI(OAc)2 and iodine, undergo transannular hydrogen abstraction to afford intramolecularly funcionalized compounds such as oxoindolizidines 83.
In 2017, Nagib et al.[49][50] reported a new method for the synthesis of 1,2-amino-alcohols using a variant of the Hofmann–Löffler–Freytag reaction to promote β selective C-H amination of alcohols.