Steroidal alkaloid
[1] A Steroidal alkaloid has also been found in Chonemorpha fragrans (Frangipani vine), 'chonemorphine' was used to treat intestinal infections in Wistar rats.
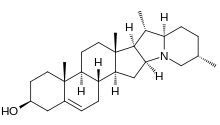
This distinctive structure is characterized by a five-membered ring formed by an amino group bonded to both the 18th and 20th carbon atoms.
The largest group consists of pentacyclic Buxus steroid alkaloids, featuring a core structure based on 4,4,14-trimethyl-9,19-cyclopregnan.
One notable example is samandarin (see figure), which may serve as the primary alkaloid depending on the species, although it may not be present in some other organisms at all.
[21] Alkaloids found in these plants include chaconine, solanine, solasodine, tomatidine, tomatine, and solanidine.
The characteristic test involves dissolving the compound in hot amyl alcohol or ethanol and watching for the formation of a jelly-like product as the mixture cools.
[1] Steroidal alkaloids with a solanidan backbone exhibit a distinctive bicyclic structure, which replaces the cholesterol side chain on the D-ring.
[20] Because of their actions on the cardiovascular, neuromuscular, and respiratory systems, Veratrum alkaloids have been used for the treatment of various conditions like myasthenia gravis, hypotension, and eclampsia.
[35] Steroidal alkaloids have been investigated for a wide range of potential bioactivities including antimicrobial, anti-inflammatory, anti-estrogenic, and chemotherapeutic[36] activity.
For example, solasodine antimicrobial bioactivity is accomplished by interfering with the synthesis of genetic substances in Saccharomyces cerevisiae and Prototheca wickerhamii.
Solasodine, for example, reduces interleukin-2 and -8 production whereas tomatidine inhibits specific nuclear translocation, JNK activation, as well as induce nitrous oxide synthase.
Veratrum alkaloid compounds act by attaching to voltage-gated sodium ion channels, altering their permeability.
[38] Furthermore, veratrum alkaloids block inactivation of sodium channels and lower their activation threshold so they remain open even at resting potential.
[38] As a result, sodium concentrations within the cell rise, leading to increased nerve and muscle excitability.
[39] These biochemical channels cause muscle contractions, repetitive firing of the nerves and an irregular heart rhythm caused by stimulation of vagal nerves which control the parasympathetic functions of the heart, lungs and digestive tract.