Hydrogen peroxide–urea
The compound is simply produced (on a scale of several hundred tonnes a year) by the dissolution of urea in excess concentrated hydrogen peroxide solution, followed by crystallization.
So just like hydrogen peroxide, the (erroneously) so-called adduct is an oxidizer but the release at room temperature in the presence of catalysts proceeds in a controlled manner.
[3][9][10] It is also used to relieve minor inflammation of gums, oral mucosal surfaces and lips including canker sores and dental irritation,[11] and to emulsify and disperse earwax.
[12] Carbamide peroxide is also suitable as a disinfectant, e.g. for germ reduction on contact lens surfaces or as an antiseptic for mouthwashes, ear drops or for superficial wounds and ulcers.
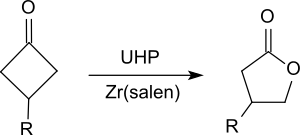
[4][13][14] It has proven to be a stable, easy-to-handle and effective oxidizing agent which is readily controllable by a suitable choice of the reaction conditions.
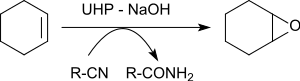
[16] It converts thiols selectively to disulfides,[15] secondary alcohols to ketones,[16] sulfides to sulfoxides and sulfones,[17] nitriles to amides,[17][18] and N-heterocycles to amine oxides.