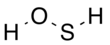Hydrogen thioperoxide
Hydrogen thioperoxide, also called oxadisulfane or sulfanol, is the chemical with the structure H–S–O–H.
Hydrogen thioperoxide has been synthesized in labs by photolysis of a mixture of ozone and hydrogen sulfide frozen in argon at 8 K[3] and by pyrolysis of di-tert-butyl sulfoxide.
[5] In the interstellar medium there is a hypothesis that hydrogen thioperoxide is formed in a reaction of sulfur monoxide with the trihydrogen cation, dihydrogen and an electron.
[9] Two molecules of hydrogen thioperoxide can undergo cyclocondensation to form sulfinothioic acid HS(=O)SH and water.
[10] Hydrosulfide HS− can react with HSOH to yield disulfane HSSH.