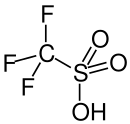
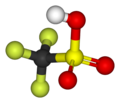
Triflic acid
[2][3] It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.
[6] Chloride ligands can be converted to the corresponding triflates: This conversion is conducted in neat HOTf at 100 °C, followed by precipitation of the salt upon the addition of ether.
Triflic acid promotes other Friedel–Crafts-like reactions including the cracking of alkanes and alkylation of alkenes, which are very important to the petroleum industry.
These triflic acid derivative catalysts are very effective in isomerizing straight chain or slightly branched hydrocarbons that can increase the octane rating of a particular petroleum-based fuel.
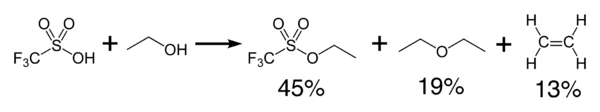
Triflic acid reacts exothermically with alcohols to produce ethers and olefins.